- +86 15383000851
- +86 15303238802
- admin@hebeianda.cn
Your Location:Home >Products >127984-74-1

pd_meltingpoint:>165°C (dec.)
Purity:99%
|
Description |
Lanreotide acetate, an octapeptide somatostatin analog, reached its first worldwide market in France for acromegaly when surgery or radiotherapy have failed to restore normal growth hormone secretion. Lanreotide is a selective inhibitor of growth hormone and reduces the secretion of growth hormone, thyrotropin, motilin and pancreatic polypeptide in humans. Lanreotide has antiproliferative properties and is reportedly in clinical trials for the prevention of restenosis following coronary artery angioplasty, for diabetic retinopathy, and as a therapy for psoriasis. Its potential for neuroendocrine tumors and hormone-responsive prostate cancer has also been demonstrated. |
|
Originator |
Beaufour-Ipsen (France) |
|
Uses |
Lanreotide acetate has been used as an inhibitor to test the plasticity of hydrogen sulfide modulation by growth hormone/thyroid hormone signaling in wild-type mice. |
|
Brand name |
Somatuline LP |
|
Biochem/physiol Actions |
Lanreotide acetate is a somatostatin analog, a selective agonist for the SRIF-1 sst2 subtype of somatostatin receptor with a binding affinity of 0.8 nM for sst2 compared to 5.2 nM for sst5, 100 nM for sst3 and more than 1000 nM for the SRIF-2 subtypes, sst1 and sst4 receptors. It is used clinically in the management of acromegaly and symptoms caused by neuroendocrine tumors, and in recent studies can also inhibit tumor growth and has shown activity against non-endocrine tumors. |
InChI:InChI=1/C54H69N11O10S2.C2H4O2/c1-29(2)45-54(75)63-44(53(74)65-46(30(3)66)47(57)68)28-77-76-27-43(62-48(69)38(56)23-32-15-18-33-10-4-5-11-34(33)22-32)52(73)60-41(24-31-16-19-36(67)20-17-31)50(71)61-42(25-35-26-58-39-13-7-6-12-37(35)39)51(72)59-40(49(70)64-45)14-8-9-21-55;1-2(3)4/h4-7,10-13,15-20,22,26,29-30,38,40-46,58,66-67H,8-9,14,21,23-25,27-28,55-56H2,1-3H3,(H2,57,68)(H,59,72)(H,60,73)(H,61,71)(H,62,69)(H,63,75)(H,64,70)(H,65,74);1H3,(H,3,4)
The present invention provides substanti...
The invention relates to an improved met...
Disclosed herein is an improved 4+4 solu...
The invention relates to an improved met...
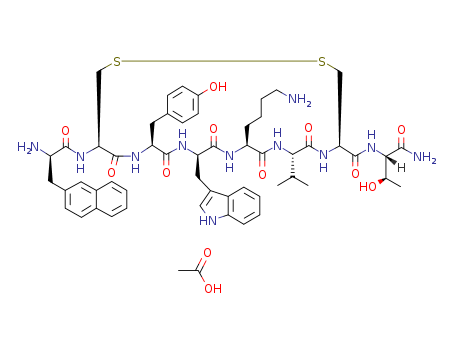
C102H115N11O14S2

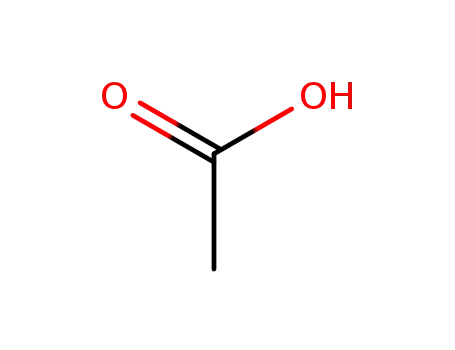
acetic acid

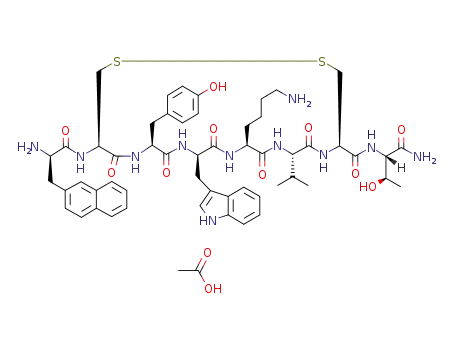
lanreotide acetate
| Conditions | Yield |
|---|---|
|
C102H115N11O14S2;
With
triethylsilane; methoxybenzene; trifluoroacetic acid; Cleland's reagent;
In
dichloromethane;
at 25 - 30 ℃;
acetic acid;
With
iodine;
In
methanol; water; acetonitrile;
at 15 - 30 ℃;
|
9.7 g |
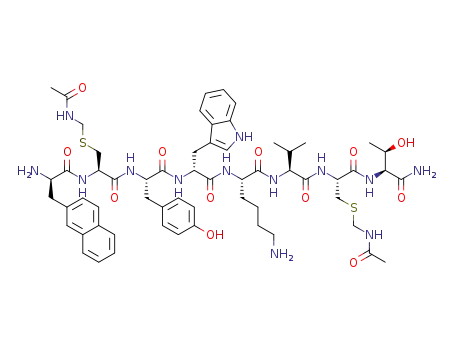
H-D-β-Nal-Cys-(Acm)-Tyr-D-Trp-Lys-Val-Cys(Acm)-Thr-NH2


acetic acid

![[cycloS-S]-3-(2-naphthyl)-D-alanyl-L-cysteinyl-L-tyrosyl-D-tryptophyl-L-lysyl-L-valyl-L-cysteinyl-L-threoninamide acetate](/upload/2023/8/d372e06d-0687-4aa7-92c7-ee9fb3f631e0.png)
[cycloS-S]-3-(2-naphthyl)-D-alanyl-L-cysteinyl-L-tyrosyl-D-tryptophyl-L-lysyl-L-valyl-L-cysteinyl-L-threoninamide acetate
| Conditions | Yield |
|---|---|
|
With
iodine;
In
methanol; water; acetonitrile;
at 15 - 30 ℃;
|
12 g |

acetic acid
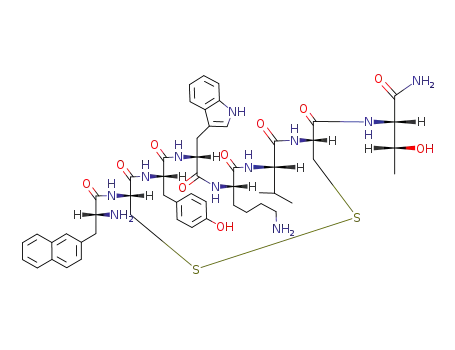
lanreotide

H-D-β-Nal-Cys-(Acm)-Tyr-D-Trp-Lys-Val-Cys(Acm)-Thr-NH2
ammonium acetate
CAS:868844-74-0
CAS:52-90-4
CAS:859-18-7
CAS:5289-74-7