- +86 15383000851
- +86 15303238802
- admin@hebeianda.cn

pd_meltingpoint:156-158 °C
Appearance:Crystalline
Purity:99%
|
Antibiotic medicines |
Lincomycin hydrochloride appears as a white crystalline powder at room temperature with slight odor or special smell and bitter taste. It is soluble in water and methanol. Feed often applied its hydrochloride salt form. The drug is the product during the growth of the population of the Streptomyces genus (Streptomyces lincolnensis), as a kind of lincosamides alkaline antibiotics. Through interaction with the 50S ribosomal subunit of the sensitive bacteria, it prevents the peptide chain elongation, thereby inhibiting protein synthesis of the bacterial cell with antimicrobial effect. However, at high concentrations, it also has bactericidal effect on the highly sensitive bacteria. Its antibacterial spectrum is the same as lincomycin but with a stronger antibacterial activity and can be subject to oral absorption quickly and completely (about 90%) with meal having little effect on the absorption. Lincomycin hydrochloride mainly has strong antibacterial effect against Gram-positive bacteria, and some anaerobic bacteria as well as Mycoplasma with its antibacterial spectrum narrower than erythromycin. Staphylococcus aureus, Streptococcus pneumoniae and Mycoplasma pneumonia Mycoplasma hyopneumoniae, and Mycoplasma gallisepticum are sensitive to the chemicals, but enterococci is generally resistant to the chemicals; anaerobic bacteria such as Bacteroides spp., Clostridium tetani, Clostridium difficile, Clostridium perfringens and Peptococcus are also sensitive to the chemicals. It is mainly used for the treatment of infections caused by Gram-positive bacteria in particular the various penicillin-resistant Gram-positive bacteria , the poultry chronic respiratory disease caused by Mycoplasma, swine enzootic pneumonia, anaerobic infections such as chicken necrotizing enterocolitis. It can also be used for the treatment of treponema dysentery, toxoplasmosis and actinomycosis of dogs and cats. Lincomycin hydrochloride has therapeutic effect when being used for the treatment of Sepsis, female genital tract, endometritis, non-gonococcal tubo-ovarian abscess, pelvic cellulitis; postoperative vaginal annex infections, peritonitis, abscesses, pneumonia, lung abscess, skin and soft tissue infections caused by most Gram-negative bacteria and some anaerobic bacteria. It can also be used for the surgical adjuvant treatment of Sepsis caused by streptococci and staphylococci, bone and joint infections, chronic bone and joint infections as well as the acute hematogenous osteomyelitis of Staphylococcus aureus. But this product can’t penetrate into cerebrospinal fluid and therefore not suitable for the treatment of meningitis. Topical administration can be applied for the treatment of Gram-positive bacteria purulent infection. |
|
Clinical application |
1. Oral formulations is suitable for treating respiratory infections, abdominal infection, female reproductive tract infections, pelvic infections, skin and soft tissue infections caused by sensitive Staphylococcus aureus and Streptococcus pneumoniae. 2. In addition to the treatment of the above infections, injected formulations are suitable for treatment of severe infections caused by streptococcus, pneumococcus and staphylococcus such as the surgical adjuvant therapy of septicemia, bone and joint infections, chronic bone and joint infections and Staphylococcus-induced acute hematogenous osteomyelitis. 3. Lincomycin hydrochloride may also be used for the treatment of infectious diseases in patients allergic to penicillin or not suitable for administration of penicillin-type drugs. Tablets: Each tablet 0.25g, 0.5g; Injection: each 0.2g, 0.6g. This information is edited by Xiongfeng Dai from Chemicalbook. |
|
Chemical Properties |
Crystalline |
|
Originator |
Lincocin,Upjohn,UK,1964 |
|
Uses |
An antibiotic produced by Streptomyces lincolnensis. Lincomycin is a lincosamide antibiotic that forms cross-links within the peptidyl transferase loop region of the 23S rRNA. Inhibits bacterial protein synthesis. Antibacterial. |
|
Manufacturing Process |
As described in US Patent 3,086,912, the process comprises cultivating Streptomyces lincolnensis var. lincolnensis in an aqueous nutrient medium containing a source of assimilable carbohydrate and assimilable nitrogen under aerobic conditions until substantial activity is imparted to the medium by production of lincolnensin and isolating the lincolnensin so produced. |
|
Brand name |
Lincocin (Pharmacia & Upjohn). |
|
Therapeutic Function |
Antibacterial |
|
General Description |
Chemical structure: macrolide |
|
Biochem/physiol Actions |
Mode of action: Lincomycin inhibits bacterial protein synthesis by forming cross-links within the peptidyl transferase loop region of the 23S rRNA.? Antimicrobial spectrum: Lincomycin hydrochloride is effective against gram-positive bacteria. |
|
Clinical Use |
Lincomycin is used for the treatment of infections causedby Gram-positive organisms, notably staphylococci, β-hemolyticstreptococci, and pneumococci. It is absorbed moderatelywell orally and distributed widely in the tissues.Effective concentrations are achieved in bone for the treatmentof staphylococcal osteomyelitis but not in the cerebrospinalfluid for the treatment of meningitis. At one time,lincomycin was considered a nontoxic compound, with alow incidence of allergy (rash) and occasional GI complaints(nausea, vomiting, and diarrhea) as the only adverseeffects. Recent reports of severe diarrhea and the developmentof pseudomembranous colitis in patients treated withlincomycin (or clindamycin), however, have necessitatedreappraisal of the role of these antibiotics in therapy. In anyevent, clindamycin is superior to lincomycin for thetreatment of most infections for which these antibiotics areindicated.Lincomycin hydrochloride occurs as the monohydrate, awhite, crystalline solid that is stable in the dry state. It isreadily soluble in water and alcohol, and its aqueous solutionsare stable at room temperature. It is degraded slowlyin acid solutions but is absorbed well from the GI tract.Lincomycin diffuses well into peritoneal and pleural fluidsand into bone. It is excreted in the urine and the bile. It isavailable in capsule form for oral administration and inampules and vials for parenteral administration. |
InChI:InChI=1/C18H34N2O6S.ClH/c1-5-6-10-7-11(20(3)8-10)17(25)19-12(9(2)21)16-14(23)13(22)15(24)18(26-16)27-4;/h9-16,18,21-24H,5-8H2,1-4H3,(H,19,25);1H/t9-,10-,11+,12-,13+,14+,15-,16-,18-;/m1./s1
A suppository composition and method for...

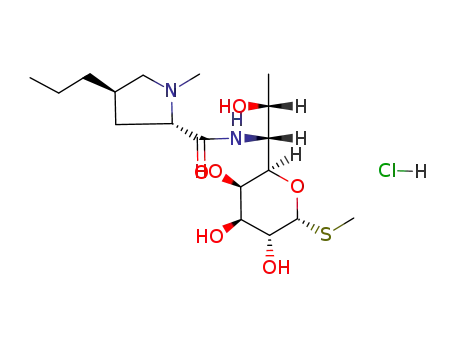
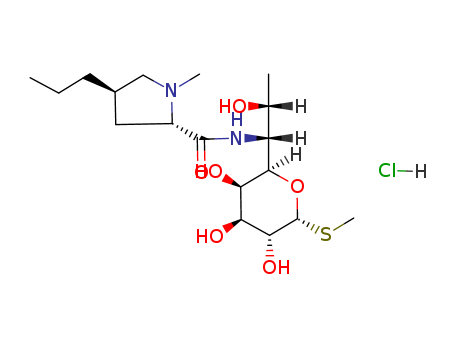
lincomycin hydrochloride
| Conditions | Yield |
|---|---|
|
|
|
|
|

lincomycin hydrochloride

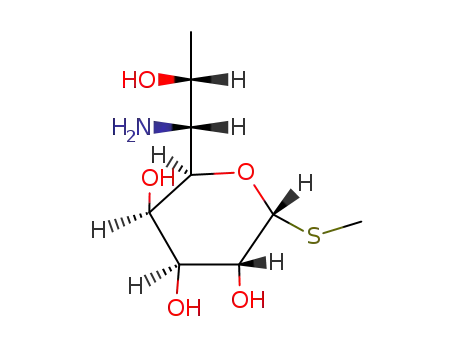
Methyl 6-Amino-6,8-dideoxy-1-thio-D-erythro-α-D-galacto-octopyranoside
| Conditions | Yield |
|---|---|
|
With
water;
at 100 ℃;
for 72h;
Inert atmosphere;
|
88% |
|
With
amerlyst A-26 hydroxide form;
In
water;
for 96h;
Reflux;
Inert atmosphere;
|
79% |
|
With
hydrazine hydrate;
at 120 ℃;
for 24h;
|
59% |

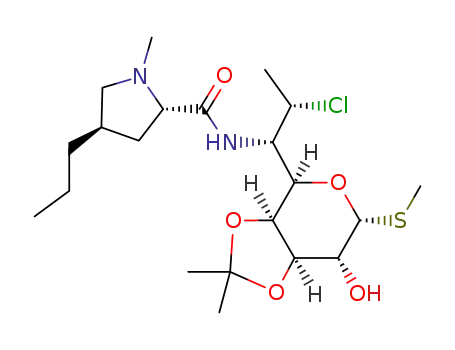
Methyl 6-Amino-6,8-dideoxy-1-thio-D-erythro-α-D-galacto-octopyranoside
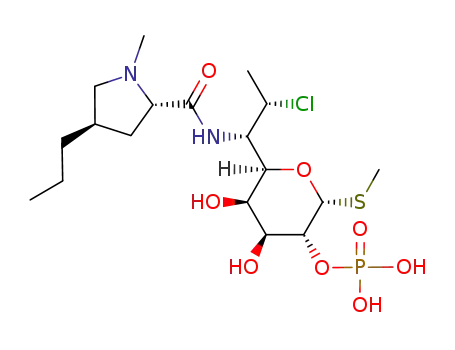
clindamycin phosphate
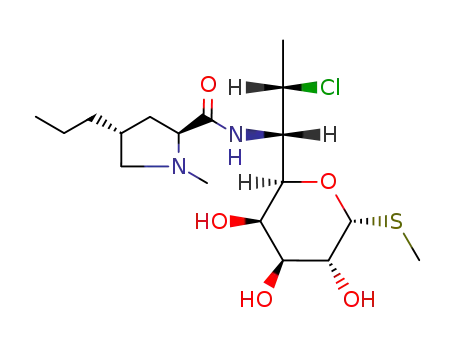
clindamycin
C21H37ClN2O5S
CAS:868844-74-0
CAS:52-90-4
CAS:24305-27-9
CAS:127984-74-1