- +86 15383000851
- +86 15303238802
- admin@hebeianda.cn
Your Location:Home >Products >Biochemical Engineering >52-90-4
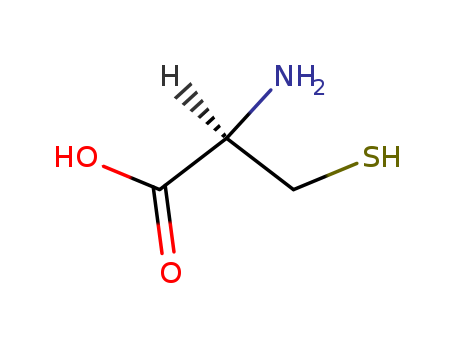

pd_meltingpoint:220 °C
Appearance:white crystalline powder
Purity:99%
|
Description |
L-Cysteine is one of the 20 natural amino acids, and it is unique among amino acids due to its sulfur content. This thiol-containing, non-essential amino acid can be oxidized to form cystine. It plays vital roles in protein synthesis, detoxification, and various metabolic functions in the human body. L-Cysteine is important for detoxifying the body. It is essential for the building of proteins in the body. |
|
Uses |
L-Cysteine, often in the form of L-Cysteine hydrochloride, is used in the baking industry as a dough conditioner. It breaks disulfide bonds in gluten, which lowers the viscosity of the dough, making it more elastic and easier to work with. It also aids in dough rising during baking. L-Cysteine can be used as a food additive and flavor enhancer in some products. It can help in treating radiation sickness, hepatitis, liver poisoning, radiopharmaceutical poisoning, and allergic diseases. It is also used in biochemical and nutritional research. |
|
Synthesis |
L-Cysteine is often extracted from human hair, as it has a high concentration of this amino acid. Other commercial sources include hog hair and poultry feathers. It can also be synthesized through a fermentation process using E. coli or other microorganisms. L-Cysteine can be synthesized from serine or methionine in animals. |
|
Biochem/physiol Actions |
NMDA glutamatergic receptor agonist. |
|
Who Evaluation |
Evaluation year: 2004 |
InChI:InChI=1/C3H7NO2S/c4-2(1-7)3(5)6/h2,7H,1,4H2,(H,5,6)/t2-/m0/s1
Cystine was reduced by ascorbic acid to ...
Changing the ionization state of L-cysteine by raising the external pH did not significantly change the apparent affinity, transport rate, or magnitude of currents induced by L-cysteine, suggesting that both the neutral zwitterionic and anionic forms of the amino acid are transported with the same net charge stoichiometry.
L-Cysteine is naturally present in the human brain and in the environment, and is much more powerful than β-N-methylamino-L-alanine, a bicarbonate-dependent excitotoxin, which has been implicated in an adult neurodegenerative disorder endemic to Guam. Thus, the potential involvement of this common sulfur-containing amino acid in neurodegenerative processes affecting the central nervous system warrants consideration.
N,N'-di-p-nitrocarbobenzoxy-L-cystine

L-Cysteine

N-(4-methylphenyl)hydroxylamine
| Conditions | Yield |
|---|---|
|
Hydrogenation;
|
![(S)-4-Amino-5-[(2S,3R)-2-((8R,11S)-8-carbamoyl-2,4-dihydroxy-1-methyl-10,14-dioxo-5,7,8,9,10,11,12,14-octahydro-13-oxa-6-thia-9-aza-benzocyclododecen-11-ylcarbamoyl)-3-hydroxy-pyrrolidin-1-yl]-5-oxo-pentanoic acid](/upload/2023/8/2b43a456-5140-458d-ba27-8bf0370e4e94.png)
(S)-4-Amino-5-[(2S,3R)-2-((8R,11S)-8-carbamoyl-2,4-dihydroxy-1-methyl-10,14-dioxo-5,7,8,9,10,11,12,14-octahydro-13-oxa-6-thia-9-aza-benzocyclododecen-11-ylcarbamoyl)-3-hydroxy-pyrrolidin-1-yl]-5-oxo-pentanoic acid

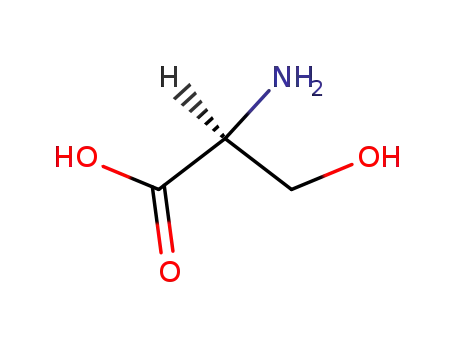
L-serin


L-Cysteine

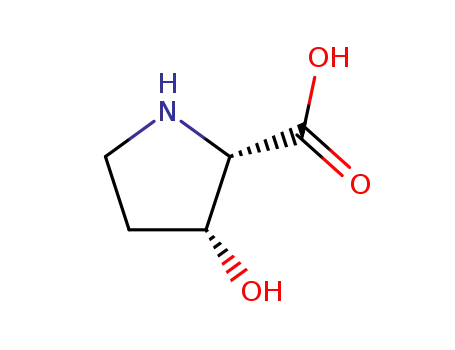
(2S,3R)-3-hydroxyproline
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; In hydrogenchloride; for 24h; product separation and chiral analysis by HPLC;
|
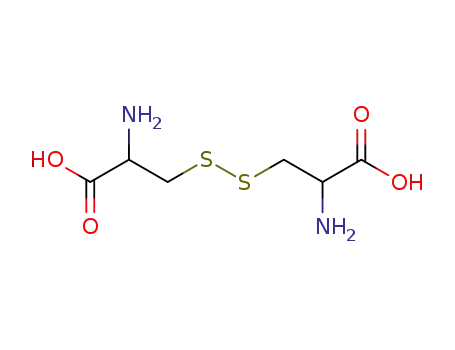
cysteine disulfide
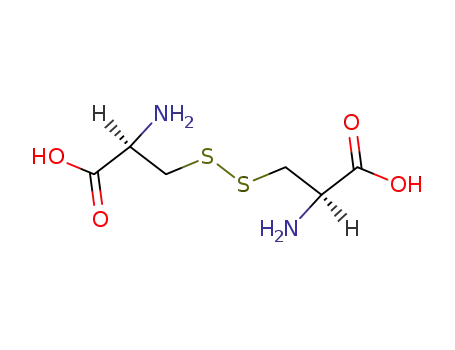
L-cystine
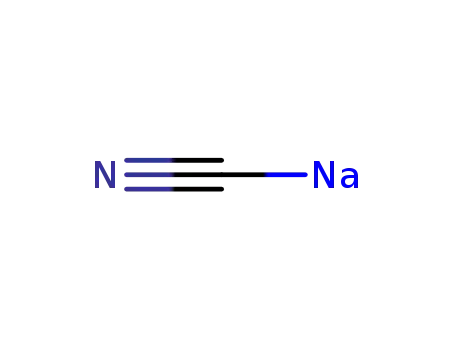
sodium cyanide
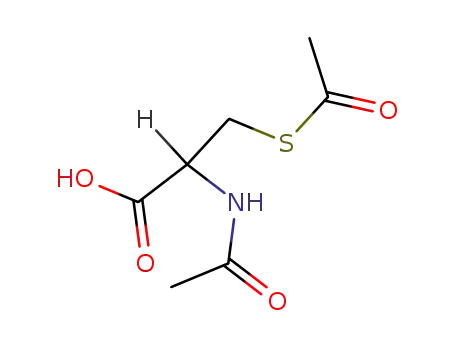
N,S-diacetyl-cysteine
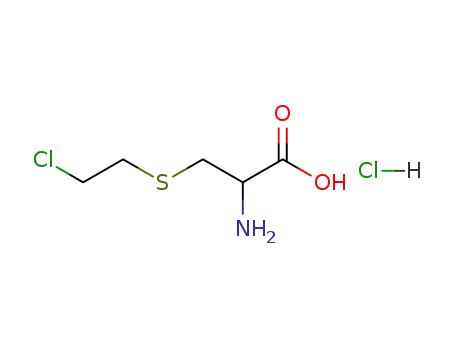
(RS)-2-amino-3-<(2-chloroethyl)sulfanyl>propanoic acid hydrochloride
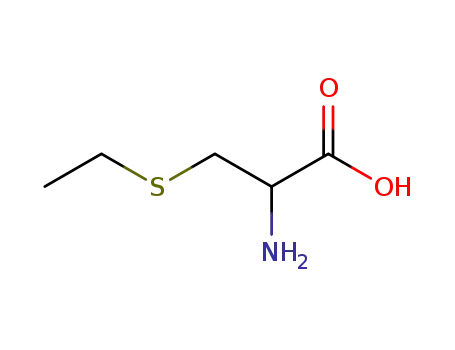
2-amino-3-(ethylthio)propionic acid
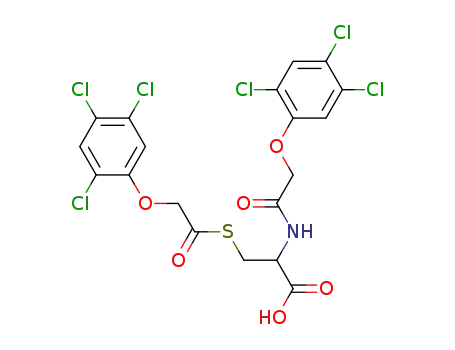
N,S-bis-[(2,4,5-trichloro-phenoxy)-acetyl]-cysteine
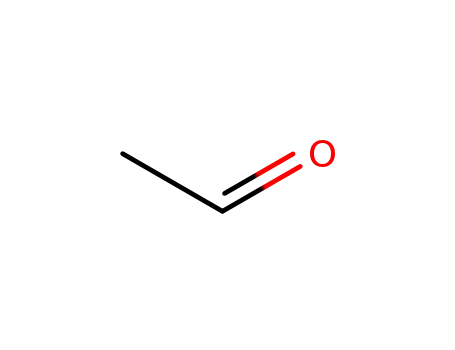
acetaldehyde
CAS:868844-74-0
CAS:12629-01-5
CAS:120-61-6
CAS:23454-33-3