- +86 15383000851
- +86 15303238802
- admin@hebeianda.cn
Your Location:Home >Products >Organic Chemistry >1119-51-3
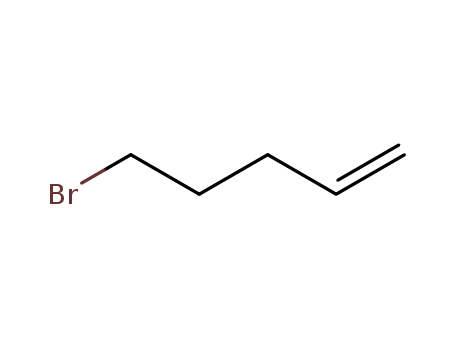
pd_meltingpoint:-106.7°C (estimate)
Appearance:clear colourles to yellow liquid
Purity:99%
|
Chemical Properties |
Clear colorless to yellow liquid |
|
Uses |
5-Bromo-1-pentene is widely utilized in the stereoselective synthesis of 7alpha-(3-carboxypropyl) estradiol. It plays an important role as starting material in the synthesis of a variety of compounds including DL-histrionicotoxin and benzophenone containing fatty acids. |
|
Synthesis Reference(s) |
Tetrahedron, 28, p. 675, 1972 DOI: 10.1016/0040-4020(72)84031-6 |
InChI:InChI=1/C5H9Br/c1-2-3-4-5-6/h2H,1,3-5H2
-
The preparation of seven-membered carboc...
Well ordered bridged organosilica highly...
Sodium allylvinylphosphinate (1) rearran...
Transition-metal-catalyzed hydroaminoalk...
Spherosilicates and polyhedral oligomeri...
The stereochemical outcomes of reactions...
(Chemical Equation Presented) 1,10-Dimet...
-
-
-
We report synthetic methodology allowing...
N, N - dimethylformamide is used as a st...
Organic transformations mediated by phot...
1,5-dibromo-pentane

bromopentene
| Conditions | Yield |
|---|---|
|
With
N,N,N,N,N,N-hexamethylphosphoric triamide;
In
N,N-dimethyl-formamide;
at 140 ℃;
for 4h;
Time;
Temperature;
Reagent/catalyst;
Large scale;
|
80.1% |
|
With
potassium tert-butylate;
In
tetrahydrofuran; toluene;
at 0 ℃;
for 0.5h;
|
69% |
|
With
N,N,N,N,N,N-hexamethylphosphoric triamide;
at 195 - 220 ℃;
|
60% |
|
With
N,N,N,N,N,N-hexamethylphosphoric triamide;
at 195 - 230 ℃;
|
59% |
|
With
N,N,N,N,N,N-hexamethylphosphoric triamide;
at 220 ℃;
for 0.0833333h;
|
57% |
|
With
N,N,N,N,N,N-hexamethylphosphoric triamide;
at 180 ℃;
|
54% |
|
With
N,N,N,N,N,N-hexamethylphosphoric triamide;
at 195 - 200 ℃;
|
47% |
|
With
18-crown-6 ether; potassium hydroxide;
at 200 ℃;
for 7h;
under 270.027 Torr;
|
47% |
|
In
N,N,N,N,N,N-hexamethylphosphoric triamide;
195 deg C then 220 deg C;
|
46% |
|
With
N,N,N,N,N,N-hexamethylphosphoric triamide;
at 205 ℃;
for 0.0833333h;
|
44% |
|
With
N,N,N,N,N,N-hexamethylphosphoric triamide;
at 195 - 220 ℃;
for 0.0833333h;
|
42% |
|
With
18-crown-6 ether; potassium tert-butylate;
In
diethyl ether;
for 1h;
|
37% |
|
With
N,N,N,N,N,N-hexamethylphosphoric triamide;
at 195 ℃;
|
|
|
With
N,N,N,N,N,N-hexamethylphosphoric triamide;
Heating;
|
|
|
With
18-crown-6 ether; potassium tert-butylate;
In
diethyl ether;
|
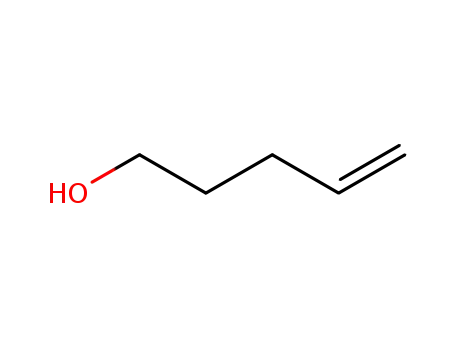
n-Pent-4-enyl alcohol

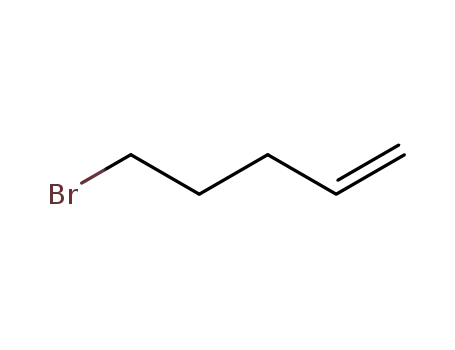
bromopentene
| Conditions | Yield |
|---|---|
|
With
carbon tetrabromide; triphenylphosphine;
In
dichloromethane;
at 0 - 20 ℃;
for 2.5h;
|
80% |
|
With
pyridine; bromine; triphenylphosphine;
In
benzene;
|
73% |
|
With
pyridine; phosphorus tribromide;
1.) -5 deg C, 30 min, 2.) RT, 2 h;
|
60% |
|
n-Pent-4-enyl alcohol;
With
phosphorus tribromide;
In
diethyl ether;
at -30 - 20 ℃;
for 16.5h;
With
sodium bromide;
In
diethyl ether;
for 21h;
|
60% |
|
With
phosphorus tribromide;
In
n-heptane;
at -10 ℃;
for 2h;
|
55% |
|
With
phosphorus tribromide;
In
Petroleum ether;
at -20 ℃;
|
46% |
|
With
pyridine; phosphorus tribromide;
at -30 - -25 ℃;
weniger gut bei Kuehlung mit Eis;
|
|
|
With
pyridine; phosphorus tribromide;
|
|
|
|
|
|
With
phosphorus tribromide;
|
|
|
With
pyridine; phosphorus tribromide;
at -5 ℃;
for 0.25h;
|
|
|
With
pyridine; phosphorus tribromide;
at -30 - -25 ℃;
for 1.16667h;
|
|
|
With
pyridine; phosphorus tribromide;
In
diethyl ether;
|
|
|
With
pyridine; triphenylphosphine dibromide 1:1 addition complex;
In
dichloromethane;
|
|
|
With
bromine; triphenylphosphine;
Yield given;
1.) CH2Cl2, 15 min., 2.) pyridine, ambient. temp., 1 h;
|
|
|
With
pyridine; phosphorus tribromide;
In
Petroleum ether;
at 0 ℃;
for 1h;
|
|
|
With
pyridine; phosphorus tribromide;
|
|
|
With
N-Bromosuccinimide; triphenylphosphine;
In
N,N-dimethyl-formamide;
at 20 ℃;
|
|
|
Multi-step reaction with 2 steps
1: KOH / diethyl ether / 2 h / 10 - 15 °C
2: LiBr / acetone / 1 h / Heating
With
potassium hydroxide; lithium bromide;
In
diethyl ether; acetone;
|
|
|
With
phosphorus tribromide;
In
diethyl ether;
at -15 - 20 ℃;
for 1.5h;
Reflux;
Inert atmosphere;
Schlenk technique;
|
|
|
With
carbon tetrabromide; triphenylphosphine;
In
dichloromethane;
|

n-Pent-4-enyl alcohol
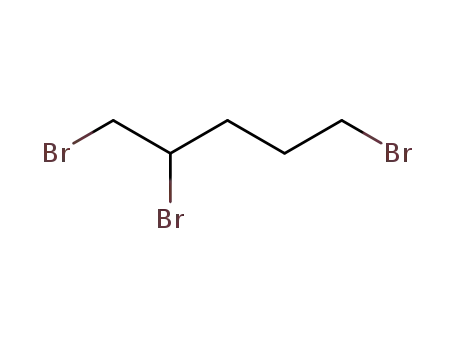
1,2,5-tribromopentane
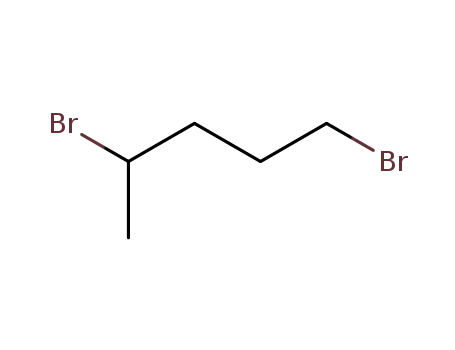
1,4-dibromopentane

1,5-dibromo-pentane
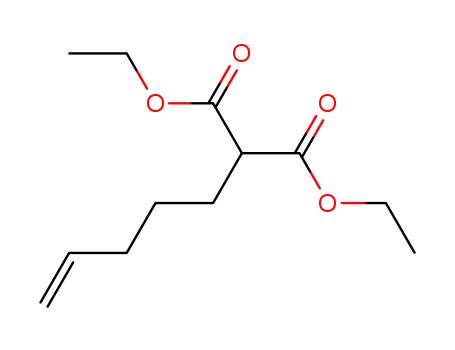
diethyl (4-pentenyl)malonate
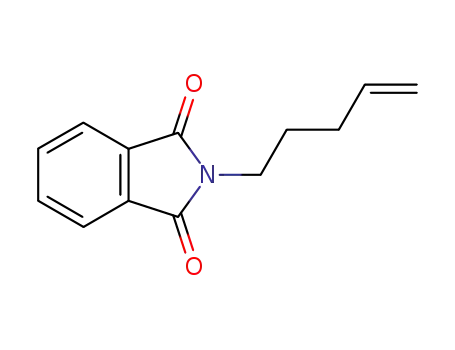
2-(pent-4-enyl)-isoindoline-1,3-dione
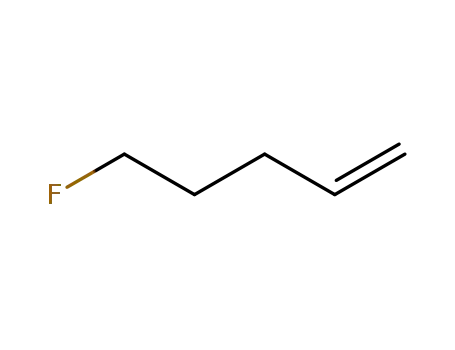
5-fluoro-pent-1-ene
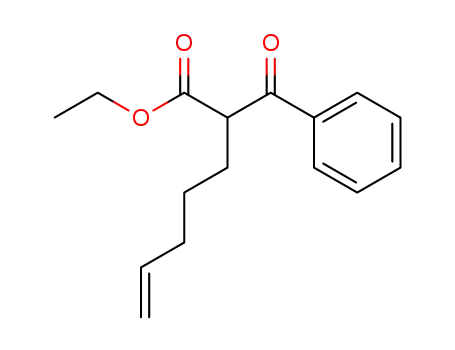
2-benzoylhept-6-enoic acid ethyl ester
CAS:5197-95-5
CAS:71368-80-4
CAS:37148-48-4
CAS:718-08-1