- +86 15383000851
- +86 15303238802
- admin@hebeianda.cn
Your Location:Home >Products >Surfactant >56-37-1
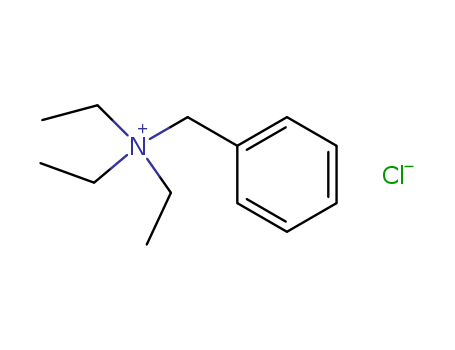

pd_meltingpoint:239 °C (dec.)(lit.)
Appearance:white to light yellow crystallize powder
Purity:99%
|
Chemical Properties |
white to light yellow crystal powder. soluble in water, aqueous solutions conduct electricity. It is hydrochloride salt of benzyltriethylammonium which acts as a phase-transfer catalyst in chemical reactions. |
|
Uses |
Benzyltriethylammonium chloride is used as a catalyst in the preparation of 2-phenylbutyronitrile from phenyl acetonitrile. It is involved in the Knoevenagel condensation of carbonyl compounds with active methylene compounds to give olefinic products. It acts as a phase transfer catalyst used in the alkylation reaction. It reacts with 1H-Pyridine-2-thione to get 2-Benzylsulfanyl-pyridine. |
|
Application |
Benzyltriethylammonium chloride is a lipophilic phase-transfer catalyst that can be used in phase-transfer catalysis (PTC) to catalyze polycondensation reactions to form high molecular weight polymers under bi-phasic conditions.It can also be used:To activate hydroxyapatite and natural phosphate for use as a solid support for Knoevenagel condensation and Claisen-Schmidt condensation, respectively at room temperature and in the absence of a solvent.To increase the efficiency of mCPBA oxidation of sulfonimine generated from arenesulfonamide and diethyl acetal of an aromatic aldehyde to form 2-sulfonyloxaziridines.In combination with antimony(V) chloride to form a catalytic system for the Friedel-Crafts acylation reactions of arenes with acyl and sulfonyl chlorides. |
|
Preparation |
Benzyltriethylammonium chloride synthesis: Add benzyl chloride, triethylamine and acetone into the reaction pot, and reflux at 63-64°C for 8 hours. Slowly lowered to 15°C, filtered, and the filter cake was washed with acetone and dried to obtain benzyltriethylammonium chloride. Yield 68.9%. |
|
Safety Profile |
Poison by intravenous route.When heated to decomposition it emits toxic vapors ofNOx and Cl. |
InChI:InChI=1/C13H22N.ClH/c1-4-14(5-2,6-3)12-13-10-8-7-9-11-13;/h7-11H,4-6,12H2,1-3H3;1H/q+1;/p-1
Nickel oxide nanoparticles, readily avai...
PROBLEM TO BE SOLVED: To provide a metho...
Thermal decomposition of hexafluorophosp...
Jojoba oil is of immense importance for ...
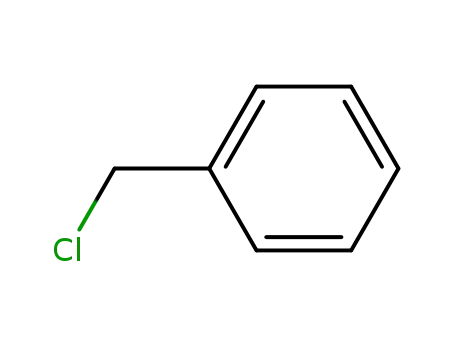
benzyl chloride


triethylamine

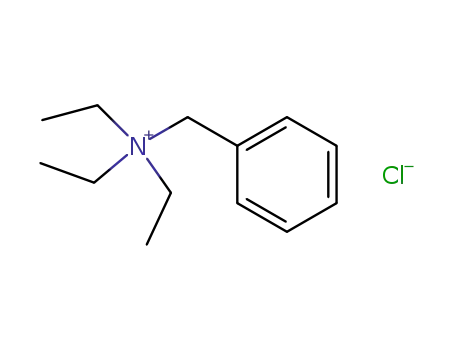
N-benzyl-N,N,N-triethylammonium chloride
| Conditions | Yield |
|---|---|
|
at 20 ℃;
|
100% |
|
In
acetonitrile;
for 1h;
Reflux;
|
95% |
|
|
75% |
|
In
acetone;
Reflux;
|
72% |
|
NiO nanoparticles;
at 100 ℃;
for 10h;
|
70% |
|
at 100 ℃;
|
|
|
With
acetone;
|
|
|
In
ethanol;
Heating;
|
|
|
|
|
|
In
ethyl acetate; N,N-dimethyl-formamide;
for 1h;
Heating;
|
|
|
In
acetone;
for 4h;
Heating;
|
|
|
at 70 ℃;
|
|
|
In
acetonitrile;
|
|
|
at 120 ℃;
for 0.0833333h;
Green chemistry;
|
|
|
In
ethanol;
for 2h;
Reflux;
|
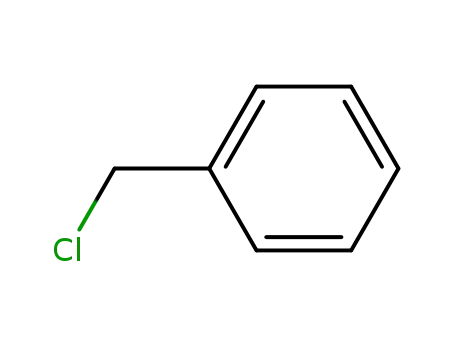
benzyl chloride


N-benzyl-N,N,N-triethylammonium chloride
| Conditions | Yield |
|---|---|
|
In
polydimethylsiloxane;
|
76.3% |
benzyl chloride

triethylamine
triethylbenzylammonium 2,4-dinitrophenoxide
4-Nitro-phenolatebenzyl-triethyl-ammonium;
benzyltriethylammonium hydroxide
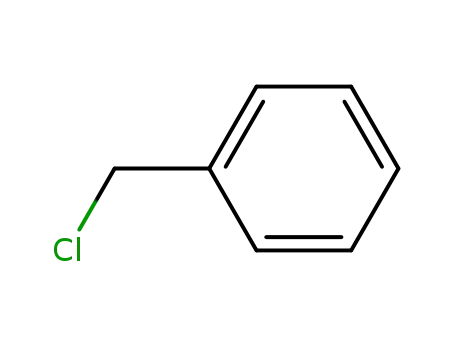
benzyl chloride

triethylamine
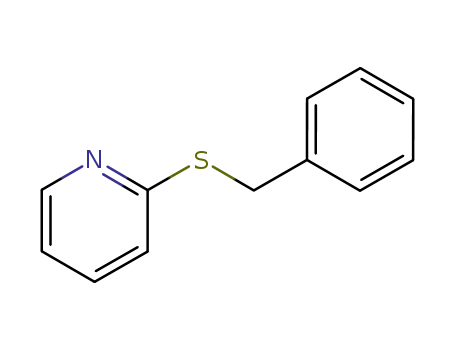
2-(benzylthio)pyridine
CAS:23239-88-5
CAS:73-78-9
CAS:167114-91-2
CAS:1009-14-9