- +86 15383000851
- +86 15303238802
- admin@hebeianda.cn
Your Location:Home >Products >API >23239-88-5

pd_meltingpoint:208oC
Appearance:hite crystalline powder
Purity:99%
|
Description |
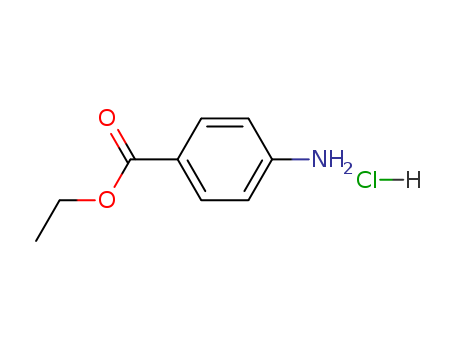
Benzocaine is a local anesthetic drug. It is directly applied to the skin and used as a topical pain reliever or in cough drops. Benzocaine works by creating a chemical barrier that halts the build up of sodium, which accumulates as the nerve endings are stimulated by pain. When sodium builds up, electrical signals also build in the nerve ending and are transmitted to the brain, which interprets the signals as pain. Benzocaine hydrochloride is a salt modification of benzocaine, formed when benzocaine is complexed with hydrochloric acid. Compared to benzocaine, benzocaine hydrochloride is more water soluble, making it more appropriate for oral administration. Benzocaine hydrochloride is usually made into powder or oil paste and used to heal wounds, ulcers, burns, skin abrasion, and hemorrhoids. Through the reduction in the excretion of ammonia and carbon dioxide of fish by benzocaine hydrochloride, the pH and alkalinity values of the transport water remains constant, thereby, benzocaine hydrochloride is used as an aid in the transport of fish. |
|
Uses |
Benzocaine Hydrochloride can be used as promising MAO inhibitors. |
InChI:InChI=1/C9H11NO2.ClH/c1-2-12-9(11)7-3-5-8(10)6-4-7;/h3-6H,2,10H2,1H3;1H
We present the synthesis and characteriz...
A series of aminoacid esters was prepare...
A compound represented by the formula (1...
In this paper, we report the synthesis a...
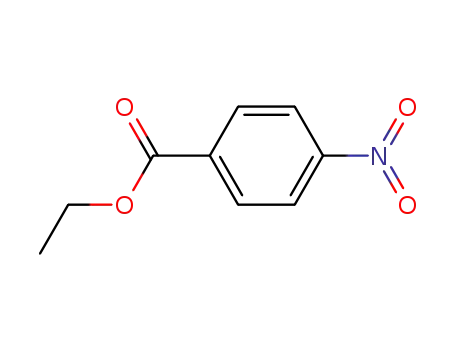
ethyl 4-nitrobenzoate

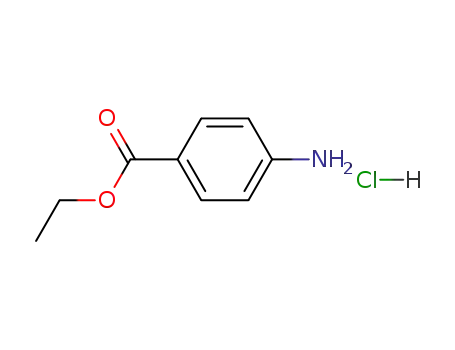
benzocaine hydrochloride
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride; hydrogen;
palladium on activated charcoal;
In
ethanol;
for 0.5h;
under 2585.7 Torr;
|
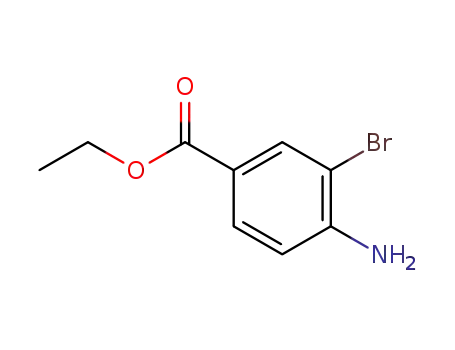
ethyl 3-bromo-4-aminobenzoate

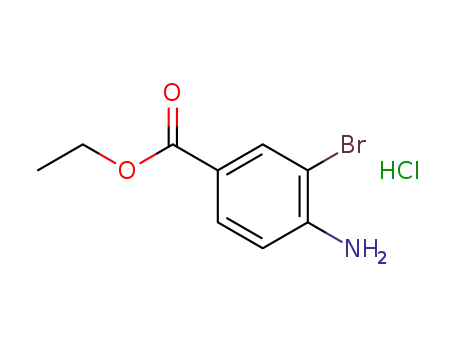
ethyl 4-amino-3-bromobenzoate hydrochloride


benzocaine hydrochloride
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride;
In
ethyl acetate;
|

ethyl 4-nitrobenzoate
ethanol
4-amino-benzoic acid

ethyl 3-bromo-4-aminobenzoate
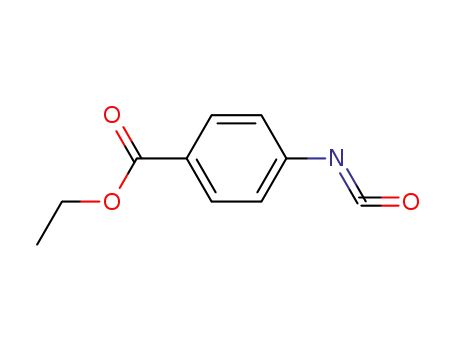
p-ethoxycarbonylphenyl isocyanate
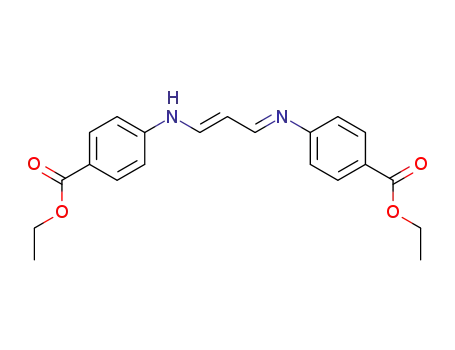
3-(4-ethoxycarbonyl-anilino)-acrylaldehyde-(4-ethoxycarbonyl-phenylimine)
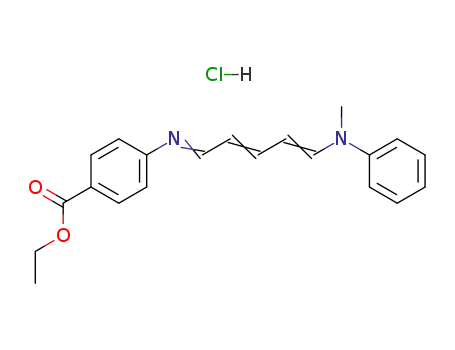
4-[5-(N-methyl-anilino)-penta-2,4-dienylidenamino]-benzoic acid ethyl ester; hydrochloride
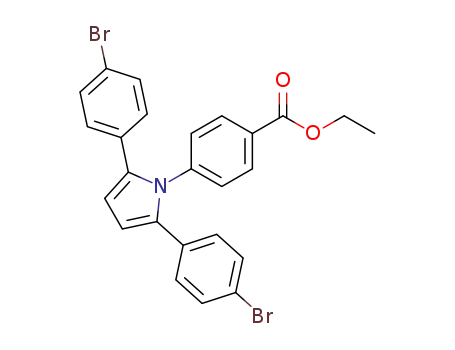
ethyl 1-p-benzoate-2,5-di-p-bromopyrrole
CAS:73-78-9
CAS:633-65-8
CAS:94-07-5
CAS:94-24-6