- +86 15383000851
- +86 15303238802
- admin@hebeianda.cn
Your Location:Home >Products >API >79517-01-4

pd_meltingpoint:153-156oC
Appearance:Colourless to off-white lyophilised solid
Purity:99%
|
Digestive system drugs |
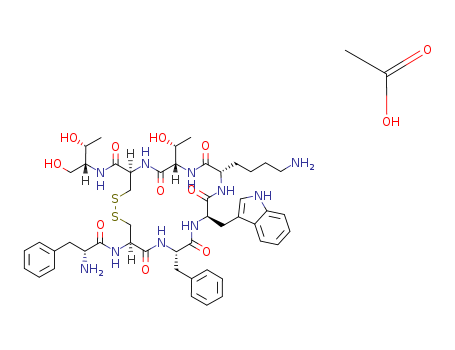
Octreotide belongs to digestive system drugs, which is an artificially synthetic cyclic octapeptide compound, and is a homolog of natural somatostatin. It contains the pharmacological activity of a natural somatostatin, and has long-lasting effects (the half-lifetime of this product is 1.5-hour, while it is 2 to 3 minutes of natural somatostatin). The product selectively inhibited the secretion of growth hormone, glucagon and insulin, and the effect is stronger than natural. It is proved by animal experiments: intravenous this product for 15 minutes, the inhibition of growth hormone release is 70 times higher than the natural somatostatin, and the inhibition of insulin release is 3 times than the natural somatostatin. It has also the inhibition effect to thyrotropin, adrenocorticotropic hormone, gastrin, cholecystokinin, vasoactive intestinal polypeptide. As well as it can inhibit the secretion of gastric acid, pancreatic amylase, lipase and pepsin. It can slow down pass through time of gastrointestinal tract, which promotes the absorption of water and electrolytes. It has the contract effect of visceral blood vessels, which can reduce splanchnic blood flow, and reduce the blood flow of liver. Thus, it reduces the portal pressure and portal blood flow, and reduces excessive secretion of intestinal tract, and it may enhance the intestinal absorption of water and the Na+. The amino acids of 1,4,8 positon is unnatural amino acids on the chemical structure of the product, so it is less susceptible to enzyme hydrolysis, and it has more longer half-lifetime. It is absorbed rapidly and completely by subcutaneous injection of the product. The time to peak plasma concentration is 0.5-hour, and the volume of distribution is 0.27 L/kg. The half-lifetime is 1.5-hour, and the metabolic clearance rate is 160 ml/min. It is biphasic elimination as intravenous injection of this product, the half-lifetime of α phase and β phase are 10 minutes and 90 minutes, respectively. The plasma protein binding rate is 65%. Octreotide is clinically used for the treatment of esophageal varices caused by portal hypertension, acromegaly, gastrointestinal bleeding including cirrhosis, esophageal varices bleeding, peptic ulcer bleeding and stress ulcer bleeding, pancreatic diseases including heavy acute pancreatitis, pancreatic trauma, or pancreatic fistula after surgery, and prevention of complications after pancreas surgery. It is also used for gastrointestinal fistula, digestive endocrine tumors such as vasoactive intestinal peptide tumors, gastrinoma, glucagon tumors, ectopic ACTH syndrome, carcinoid syndrome, and it is used for acromegaly, exophthalmus hyperthyroidism disease, after the removal of the entire colon appearing persistent and intractable diarrhea, AIDS-related diarrhea. Intrathecal injection of this product can treat cancer pain. |
|
Dosage and administration |
1. prevention of complications of pancreatic surgery: one hour before surgery, subcutaneous injection of 0.1mg octreotide; then 0.1mg subcutaneously 3 times a day for seven days. 2. treatment of varices caused by esophagus portal hypertension: 0.1mg intravenous injection, then 0.5mg, every two hours by intravenous drip. 3. stress ulcers and gastrointestinal bleeding: 0.1mg subcutaneously 3 times a day. 4. severe pancreatitis: 0.1mg subcutaneous injection, 4 times a day for 3 to 7 days; pancreatic injury or pancreatic fistula after surgery, subcutaneous injection, each 0.1mg, every 8 hours for 7 to 14 days. 5. gastrointestinal fistula and digestive tract endocrine system of cancer adjuvant therapy: subcutaneous 0.1mg, 3 times a day, a course for10 to 14 days. 6. digestive system endocrine tumors, Graves' disease, acromegaly and AIDS-related diarrhea: subcutaneous 0.1mg, 3 times a day, acromegaly treatment for 10 to 14 days. There are injection site pain or tingling, and it is usually ease after 15 minutes. Gastrointestinal adverse reactions include anorexia, nausea, vomiting, diarrhea, abdominal cramps. Occasionally it will appear hyperglycemia, cholelithiasis, abnormal glucose tolerance, and liver function abnormalities (γ-glutamyl transferase increased and transaminase slightly increased) and others. Patients with dysfunction of kidney and pancreatic, and cholelithiasis should be used with caution. Pregnant women, lactating women and children are prohibition. The above information is edited by the chemicalbook of Kui Ming. |
|
Category |
toxic substances |
|
Toxicity grading |
highly toxic |
|
Acute toxicity |
intravenous-rat LD50: 18.1 mg/kg; intravenous-mouse LD50: 72.3 mg/kg |
|
Flammability hazard characteristics |
combustible; fire decomposition of toxic nitrogen oxide compounds, sulfur oxide fumes |
|
storage properties |
cold warehouse, ventilated and dry; and separately stored with food raw materials |
|
Extinguishing media |
water, carbon dioxide, dry power, sandy soil |
|
Chemical Properties |
Colourless to off-white lyophilised solid |
|
Originator |
Sandostatin,Novartis Pharma AG,Switz. |
|
Uses |
Octapeptide analog of Somatostatin. A gastric antisecretory agent. Used in treatment of acromegaly |
|
Indications |
Octreotide (Sandostatin) is a synthetic somatostatin analogue. It is used in a variety of situations and must be given subcutaneously or intravenously. Most commonly, it is used as a continuous intravenous infusion in patients hospitalized with bleeding varices, because it decreases splanchnic circulation and therefore reduces portal pressures.A long-acting depot form is approved for the suppression of severe diarrhea and flushing associated with malignant carcinoid syndrome and for the treatment of the profuse watery diarrhea associated with vasoactive intestinal peptide tumor. |
|
Brand name |
Sandostatin (Novartis). |
|
Therapeutic Function |
Antiulcer, Growth hormone inhibitor |
|
General Description |
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards |
|
Clinical Use |
Relief of symptoms of gastro-enteropancreatic endocrine tumours and acromegaly |
|
Safety Profile |
A poison by subcutaneous andintravenous routes. Human systemic effects: changes instructure or function of endocrine pancreas, liver functiontests impaired. When heated to decomposition it emitstoxic vapors of NOx and SOx |
|
Drug interactions |
Potentially hazardous interactions with other drugs Ciclosporin: ciclosporin concentration reduced. |
|
Metabolism |
Extensive hepatic metabolism.1 |
InChI:InChI=1/C49H66N10O10S2/c1-28(61)39(25-60)56-48(68)41-27-71-70-26-40(57-43(63)34(51)21-30-13-5-3-6-14-30)47(67)54-37(22-31-15-7-4-8-16-31)45(65)55-38(23-32-24-52-35-18-10-9-17-33(32)35)46(66)53-36(19-11-12-20-50)44(64)59-42(29(2)62)49(69)58-41/h3-10,13-18,24,28-29,34,36-42,52,60-62H,11-12,19-23,25-27,50-51H2,1-2H3,(H,53,66)(H,54,67)(H,55,65)(H,56,68)(H,57,63)(H,58,69)(H,59,64)/t28-,29?,34-,36+,37+,38-,39-,40+,41+,42+/m1/s1
Disclosed herein is an improved 4+4 solu...
Stable, biologically compatible pharmace...
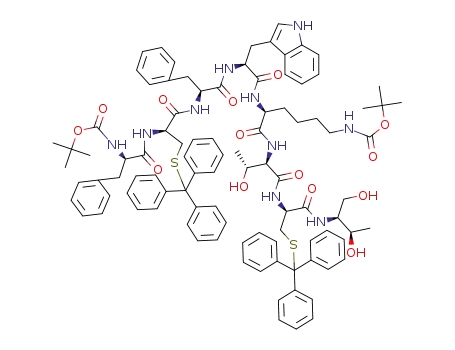
Boc-D-Phe-Cys(Trt)-Phe-D-Trp-Lys(Boc)-Thr(OtBu)-Cys(Trt)-Thr-OH

acetic acid

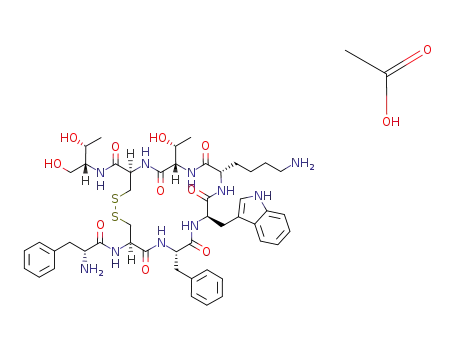
octreotide acetate
| Conditions | Yield |
|---|---|
|
Boc-D-Phe-Cys(Trt)-Phe-D-Trp-Lys(Boc)-Thr(OtBu)-Cys(Trt)-Thr-OH; With triethylsilane; methoxybenzene; trifluoroacetic acid; In dichloromethane; at 10 - 30 ℃;
acetic acid; With iodine; In methanol; water; acetonitrile; at 25 - 30 ℃;
|
30.21% |

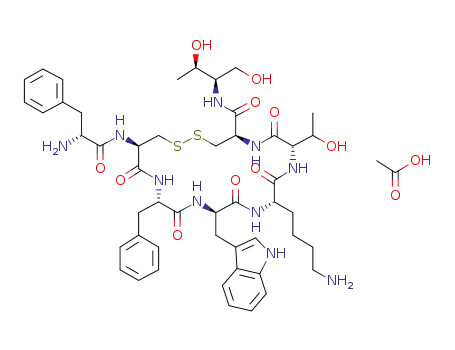
Octreotide acetate
| Conditions | Yield |
|---|---|
|
|
|
|
|

acetic acid
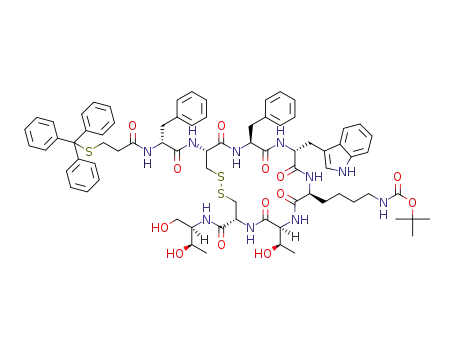
Lys-boc octreotide 3-tritylmercaptopropionamide
CAS:868844-74-0
CAS:52-90-4
CAS:29622-29-5
CAS:159634-47-6