- +86 15383000851
- +86 15303238802
- admin@hebeianda.cn
Your Location:Home >Products >Organic Chemistry >593-51-1
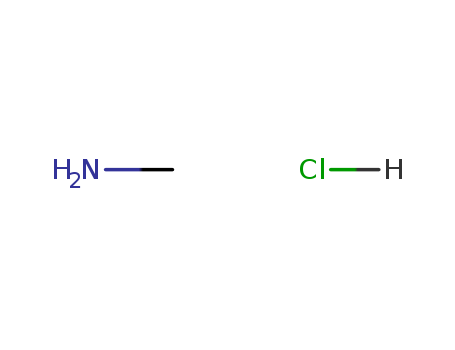
pd_meltingpoint:231-233 °C(lit.)
Appearance:white to light tan solid
Purity:99%
|
Chemical Properties |
white to light tan solid |
|
Uses |
Methylamine is used as a building block for the synthesis of other organic compounds. It is on the DEA watchlist for chemical precursors because of clandestine use in manufacture of the drug MDMA (ecstasy) and methamphetamine.The HCl salt is in the form of white deliquescent crystals, and is frequently substitutable for the aq. soln. in many synthesis preparations. Methylamines are used directly as catalysts or as raw materials to produce other compounds with catalytic activity. Fuel additives are used to improve engine performance in a variety of ways. Trimethylamine is used to make paper chemicals. The manufacture of intermediates to make pharmaceuticals is one of the most diverse uses of methylamines. |
|
Preparation |
Methylamine hydrochloride is prepared by heating a mixture of aqueous formaldehyde and ammonium chloride; the residual water and the formic acid produced in the reaction are removed via vacuum distillation. This leaves behind solid methylamine hydrochloride. The solid is then purified by organic extractions and additional vacuum distillations. Methylamine gas for the reaction with P2P is generated in situ by heating the hydrochloride salt with sodium hydroxide. Alternatively, methylamine can be liberated from the hydrochloride with base and collected in a cold finger or flask immersed in a dry ice bath. |
|
Definition |
ChEBI: The hydrochloride formed from methylamine. |
|
Purification Methods |
Crystallise the salt from n-butanol, absolute EtOH or MeOH/CHCl3. Wash it with CHCl3 to remove traces of dimethylamine hydrochloride. Dry it under vacuum first with H2SO4 then P2O5. It is deliquescent; store it in a desiccator over P2O5. [Beilstein 4 IV 122.] |
InChI:InChI=1/CH5N.ClH/c1-2;/h2H2,1H3;1H
The complexes (NO)I(Y)> (Y=NMe2 or NHR, ...
Organic-inorganic halide perovskite (OIH...
Ionization radiation photons such as X-r...
Chemically tuned inorganic-organic hybri...
Deoxygenative reduction of amides is a c...
Hydroboration of amides is a useful synt...
Novel NH4+-doped MA1?x(NH4)xPbBr3perovsk...
water

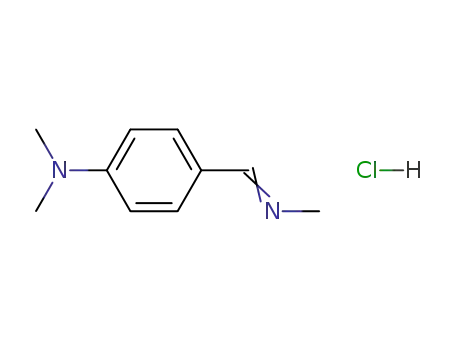
4-dimethylamino-benzaldehyde methylimine; hydrochloride

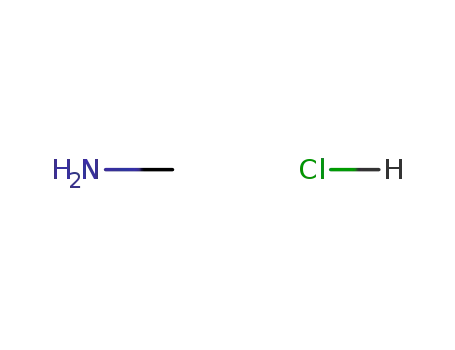
methylamine hydrochloride

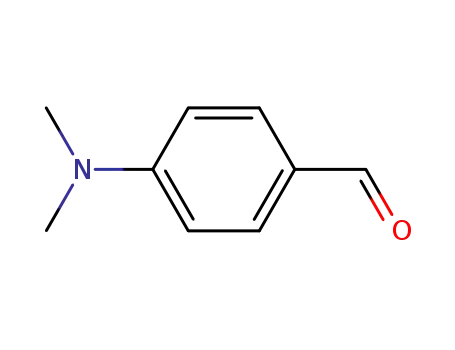
4-dimethylamino-benzaldehyde
| Conditions | Yield |
|---|---|
|
|

water


4-dimethylamino-benzaldehyde methylimine; hydrochloride

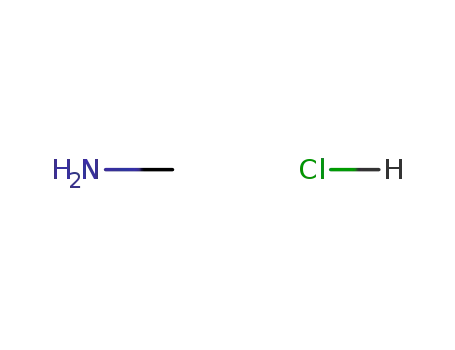
methylamine hydrochloride


4-dimethylamino-benzaldehyde
| Conditions | Yield |
|---|---|
|
|
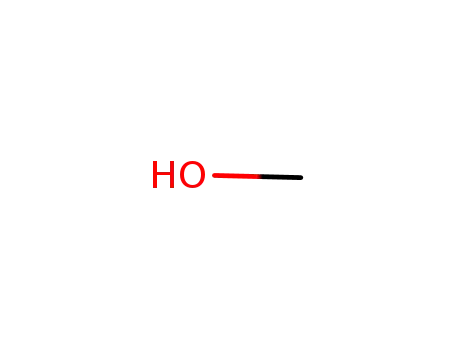
methanol
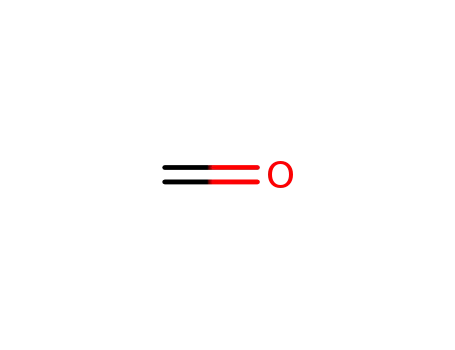
formaldehyd
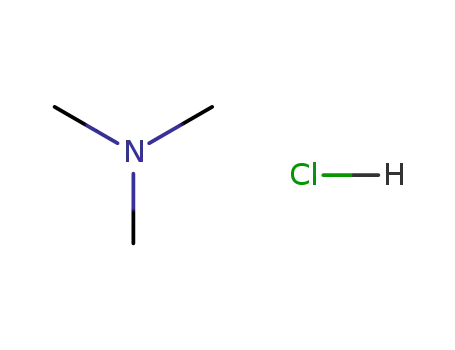
trimethylamine hydrochloride
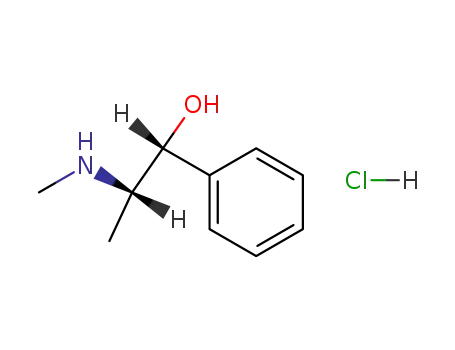
ephedrine hydrochloride
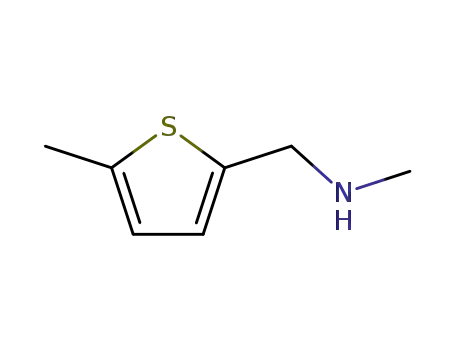
5-methyl-2-<(methylamino)methyl>thiophene
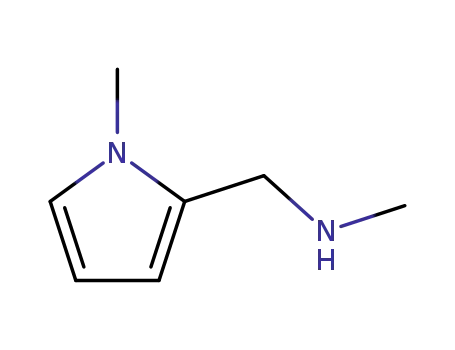
N-methyl(1-methyl-1H-pyrrol-2-yl)methylanamine
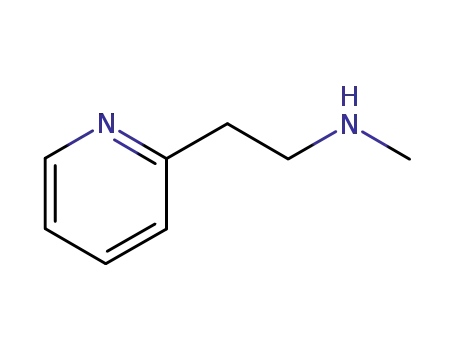
betahistine
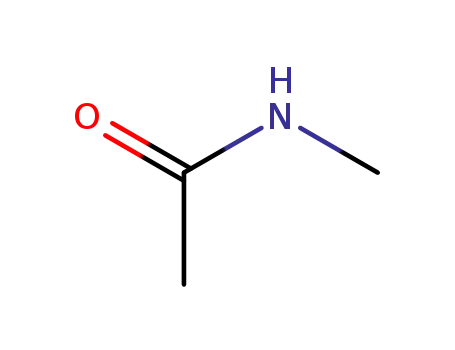
N-methyl-acetamide
CAS:23239-88-5
CAS:73-78-9
CAS:90076-65-6
CAS:280755-12-6