- +86 15383000851
- +86 15303238802
- admin@hebeianda.cn
Your Location:Home >Products >Fine Chemicals >13488-22-7
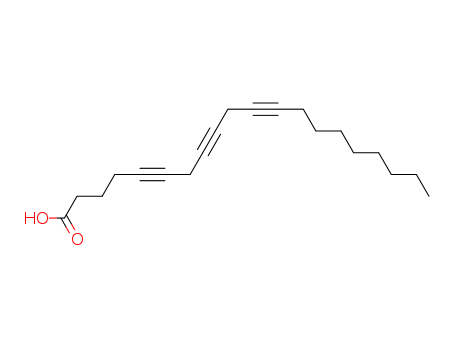

pd_meltingpoint:70.5-71.5 °C
Purity:99%
|
Uses |
ETI is a leukotriene antagonist and selective 5-LO (5-lipoxygenase) inhibitor. |
|
Definition |
ChEBI: A C20 polyunsaturated fatty acid having three triple bonds in the 5-, 8- and 11-positions. |
|
Biological Activity |
5,8,11-eicosatriynoic acid is a selective inhibitor of lipoxygenases with id50 values of 24 μm and 340 μm for n-8 lipoxygenase and fatty acid cycle-oxygenase , respectively[1][2].fatty acid cycle-oxygenase and n-8 lipoxygenase catalyze the initial reactions which lead to the formation of three major arachidonic acid metabolites in human platelets. in several tissues, these two enzymes act concomitantly on arachidonic acid [1].5,8,11-eicosatriynoic acid (5,8,11-et) is a selective inhibitor of lipoxygenases with id50 values of 24 μm and 340 μm for n-8 lipoxygenase and fatty acid cycle-oxygenase, respectively. 5,8,11-eicosatriynoic acid was useful for studies on physiological and pathophysiological roles of 12 l-hydroxy-5,8,10,14-eicosatetraenoic acid formation in various tissues [1]. in mouse mastocytoma cells, 5,8,11-eicosatriynoic acid inhibited a23187 and l-cysteine induced leukotriene c (ltc4) biosynthesis with id50 value of 5 μm [2]. |
|
references |
[1]. hammarstrm s. selective inhibition of platelet n-8 lipoxygenase by 5,8,11-eicosatriynoic acid. biochim biophys acta. 1977 jun 22;487(3):517-9. [2]. orning l, hammarstrm s. inhibition of leukotriene c and leukotriene d biosynthesis. j biol chem. 1980 sep 10;255(17):8023-6. |
InChI:InChI=1/C20H28O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20(21)22/h2-8,11,14,17-19H2,1H3,(H,21,22)
5,8,11-Eicosatriynoic acid selectively inhibited n — 8 lipoxygenase (ID50 = 24 μM) in comparison to fatty acid cyclo-oxygenase (ID50 = 340 μM) in human platelets. 5,8,11,14-Eicosatetraynoic acid inhibited the enzymes less selectively (ID50 values: 4 μM for the lipoxygenase and 8 μM for the cyclooxygenase)....
Compound, corresponding to the formula i...

1-bromoundec-2-yne

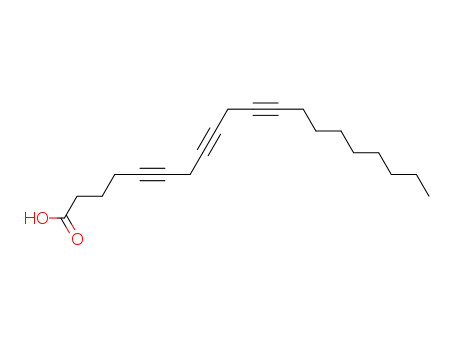
5,8,11-eicosatriynoic acid
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 3 steps
1: (i) EtMgBr, (ii) CuCl, THF, (iii) /BRN= 1816644/
2: PBr3, Py / diethyl ether
3: (i) EtMgBr, THF, (ii) CuCN, (iii) /BRN= 1817884/
With pyridine; phosphorus tribromide; In diethyl ether;
|
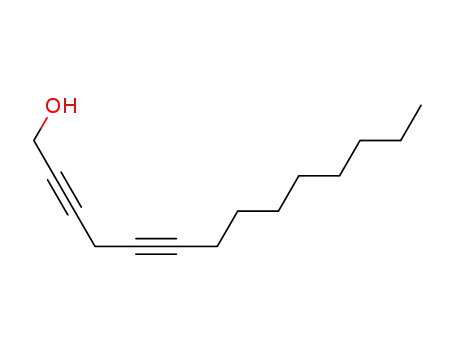
tetradeca-2,5-diyn-1-ol


5,8,11-eicosatriynoic acid
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: PBr3, Py / diethyl ether
2: (i) EtMgBr, THF, (ii) CuCN, (iii) /BRN= 1817884/
With pyridine; phosphorus tribromide; In diethyl ether;
|
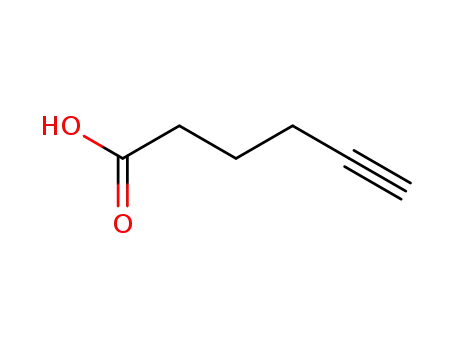
hex-5-ynoic acid
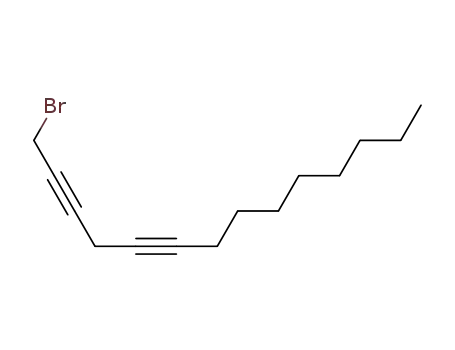
1-bromo-tetradeca-2,5-diyne

1-bromoundec-2-yne

tetradeca-2,5-diyn-1-ol
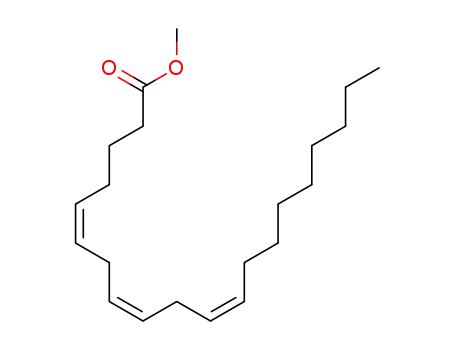
methyl 5Z,8Z,11Z eicosatrienoate
CAS:119356-77-3
CAS:868844-74-0
CAS:55981-09-4
CAS:7681-11-0