- +86 15383000851
- +86 15303238802
- admin@hebeianda.cn
Your Location:Home >Products >Chemical Reagents >96-26-4
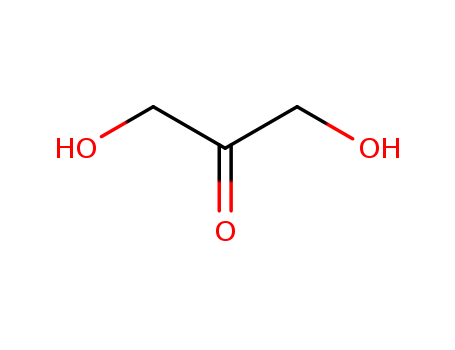

pd_meltingpoint:75-80 °C
Appearance:white powder
Purity:99%
|
Chemical Properties |
white powder |
|
Occurrence |
A derivative of naturally occurring starch |
|
Uses |
1,3-Dihydroxyacetone can be used as artificial tanning agent. |
|
Preparation |
Usually produced commercially from Bacillus macerans or Bacillus circulans fermentation of starch or starch hydrolysate |
|
Definition |
ChEBI: A ketotriose consisting of acetone bearing hydroxy substituents at positions 1 and 3. The simplest member of the class of ketoses and the parent of the class of glycerones. |
|
Taste threshold values |
Reported to have a taste threshold value lower than that of sucrose with a detection level of 3.9 to 27 ppm and a recognition level of 11 to 52 ppm. |
|
General Description |
Dihydroxyacetone (DHA) is a browning ingredient widely used in cosmetics such as sunless tanning formulations. It participates in a chemical staining reaction called Milliard reaction in which it reacts with the amino groups of proteins to result in a mixture of high molecular weight pigments.Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards. |
|
Safety Profile |
Mutation data reported. When heated to decompositionit emits acrid smoke and irritating vapors. |
|
Consumer Uses |
This substance is used in the following products: cosmetics and personal care products and perfumes and fragrances. Other release to the environment of this substance is likely to occur from: indoor use as processing aid and outdoor use as processing aid. |
InChI:InChI=1/C3H6O3/c4-1-3(6)2-5/h4-5H,1-2H2
Biocatalytic cascades afford the develop...
The selective oxidation of α,α-diglycero...
Selective oxidation of the secondary hyd...
Abstract: In the present investigation, ...
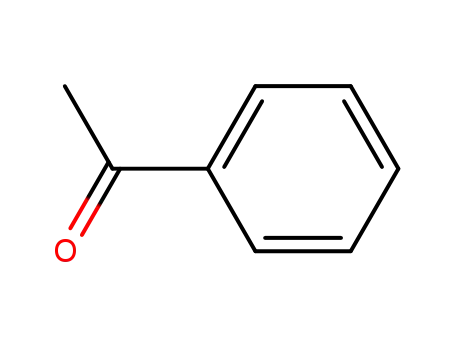
acetophenone

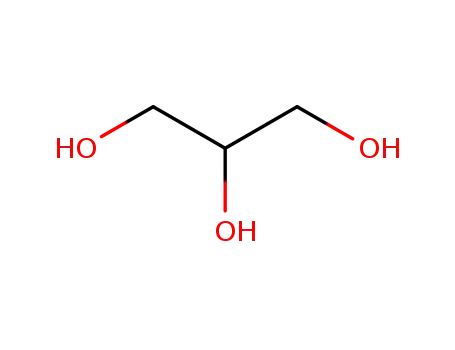
glycerol

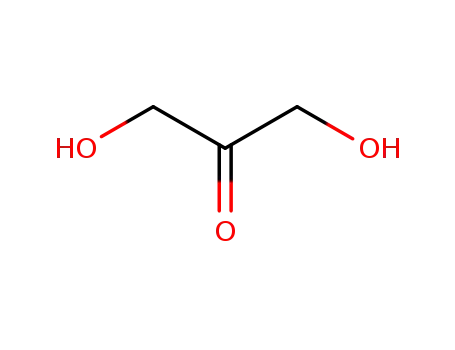
dihydroxyacetone

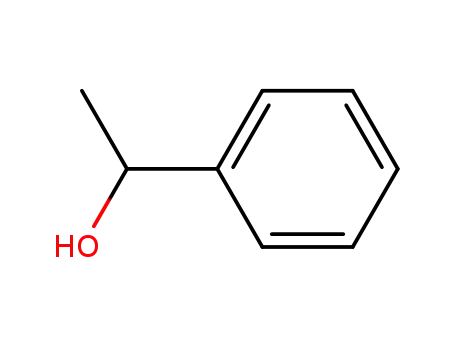
1-Phenylethanol
| Conditions | Yield |
|---|---|
|
With
[IrCl(COD)(C3H2N2(3,4,5-trimethoxybenzyl)(n-Bu))]; potassium hydroxide;
at 120 ℃;
for 7h;
Inert atmosphere;
|
80% |
|
With
C40H50IrNP2;
at 120 ℃;
for 1h;
chemoselective reaction;
Inert atmosphere;
|
8 %Chromat. 6 %Chromat. |

acetophenone


glycerol


dihydroxyacetone


1-Phenylethanol

![cis-(2-methyl-2-phenyl-[1,3]-dioxolane-4-yl)methanol](/upload/2023/8/15737b41-51c3-4309-b20d-4a067bda3af2.png)
cis-(2-methyl-2-phenyl-[1,3]-dioxolane-4-yl)methanol

![trans-(2-methyl-2-phenyl-[1,3]-dioxolane-4-yl)methanol](/upload/2023/8/bca84de9-6c4b-4ada-8aab-7d44847bd55d.png)
trans-(2-methyl-2-phenyl-[1,3]-dioxolane-4-yl)methanol

C11H14O3

C11H14O3
| Conditions | Yield |
|---|---|
|
With
bis[dichloro(pentamethylcyclopentadienyl)iridium(III)];
at 40 ℃;
for 1h;
Molecular sieve;
|
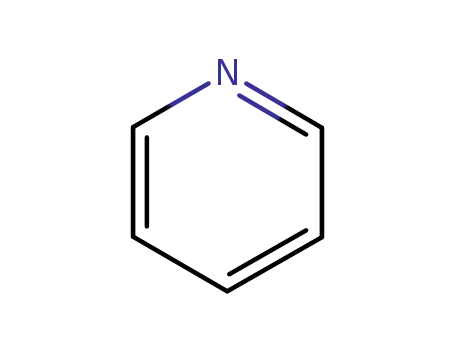
pyridine
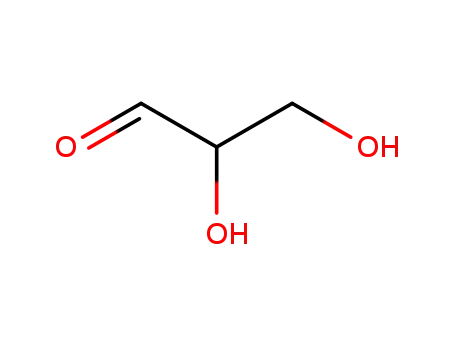
Glyceraldehyde
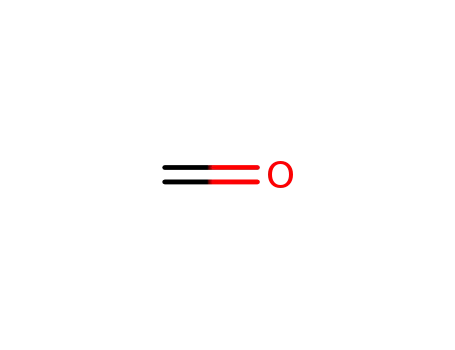
formaldehyd
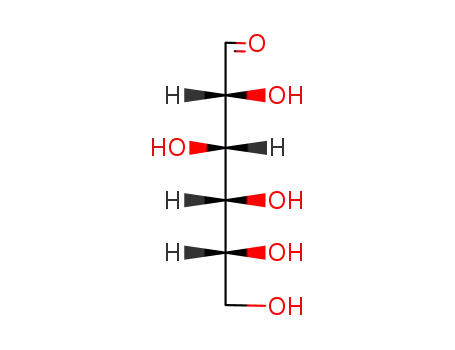
D-glucose
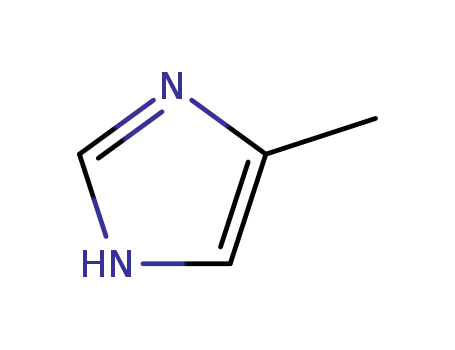
4-methyl-1H-imidazole
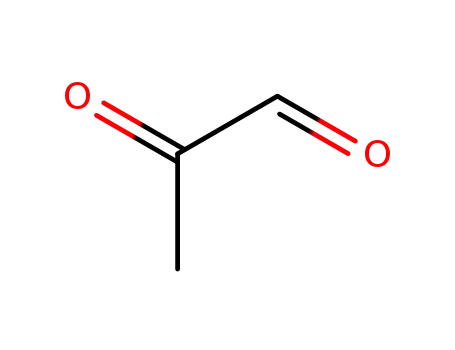
2-oxopropanal
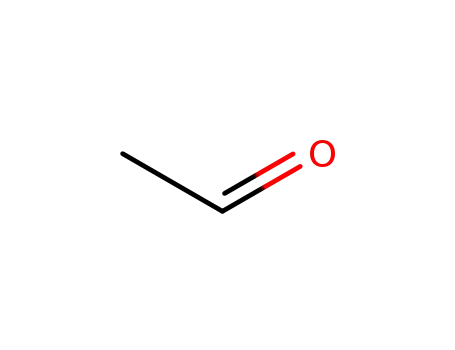
acetaldehyde
methylammonium carbonate
CAS:119356-77-3
CAS:868844-74-0
CAS:5413-05-8
CAS:1182367-47-0