- +86 15383000851
- +86 15303238802
- admin@hebeianda.cn
Your Location:Home >Products >Biochemical Engineering >99896-85-2
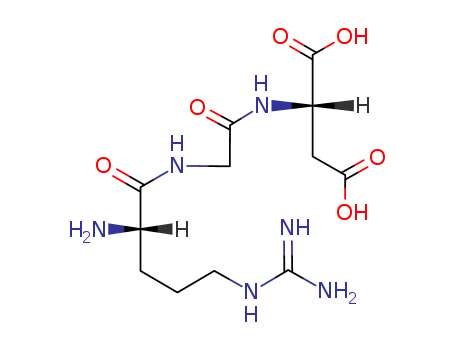

pd_meltingpoint:153-155℃ (ethyl ether )
Purity:99%
|
Chemical Properties |
White to off-white powder |
|
Uses |
Arg-Gly-Asp has been used:to saturate the cell surface Arg-Gly-Asp (RGD) receptors and inhibit marrow stromal cell (MSC) attachment to peptide modified hydrogelin adhesion assayin inhibition assay to confirm the β1-integrin-dependency of cell adhesionin textile fabrication and RGD textile functionalizationto engineer a population of human dermal fibroblasts with impaired migrationto modify glass substrate and evaluate the behavior of human bone marrow mesenchymal stem cells (hBM-MSCs)in the cell culture of human mammary epithelial cells |
|
General Description |
Arg-Gly-Asp (RGD) is an integrin binding site, which belongs to the class of adhesive proteins. It functions as a brain tumor targeting ligand. |
|
Biological Activity |
synthetic peptides containing the arginine-glycine-aspartate (rgd) were extensively used as inhibitors of integrin-ligand interactions in studies of cell adhesion, migration, growth and differentiation, since the rgd motif is an integrin-recognition motif found in many ligands. |
|
Biochem/physiol Actions |
Primary sequence involved with the binding of proteins to cell surfaces |
|
in vitro |
rgd peptide can induce apoptosis in the absence of signals and integrin-mediated cell clustering. previous study demonstrates that rgd peptides promote apoptosis through activation of conformation changes enhancing pro-caspase-3 activation and autoprocessing [1]. |
|
in vivo |
anima study suggested that the rgd-4c-fitc-peptide bound to both endothelial and tumor cells in vivo and that peptide targeting should allow the delivery of therapeutic drugs to both endothelial and tumor cells [2]. |
|
references |
[1] nature. 1999 feb 11;397(6719):534-9.rgd peptides induce apoptosis by direct caspase-3 activation. buckley cd1, pilling d, henriquez nv, parsonage g, threlfall k, scheel-toellner d, simmons dl, akbar an, lord jm, salmon m. [2] cancer res. 2002 sep 15;62(18):5139-43. arginine-glycine-aspartic acid (rgd)-peptide binds to both tumor and tumor-endothelial cells in vivo. zitzmann s1, ehemann v, schwab m. |
InChI:InChI=1/C12H22N6O6/c13-4-6(19)12(17,10(23)24)7(9(21)22)8(20)5(14)2-1-3-18-11(15)16/h5,7H,1-4,13-14,17H2,(H,21,22)(H,23,24)(H4,15,16,18)
Arg-Gly-Asp (RGD) peptides represent the...
The present invention relates to the fie...
A bivalent poly(ethylene glycol) or PEG ...
-
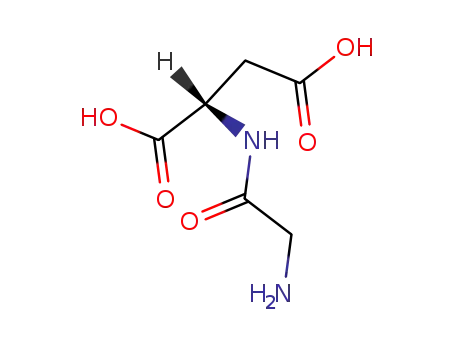
glycyl-L-aspartic acid

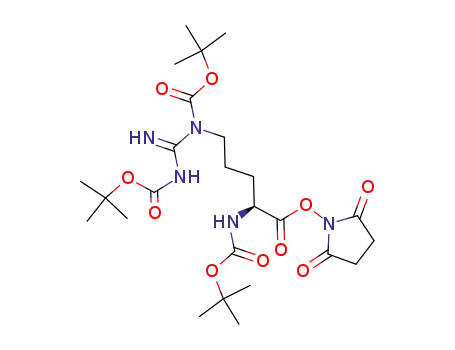
Nα,NG,NG'-tri-Boc-L-arginine N-hydroxysuccinimide ester

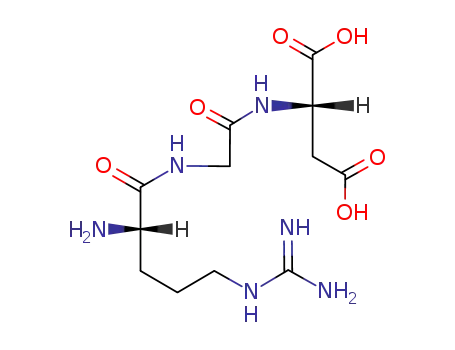
Arg-Gly-Asp
| Conditions | Yield |
|---|---|
|
glycyl-L-aspartic acid; Nα,NG,NG'-tri-Boc-L-arginine N-hydroxysuccinimide ester;
With
4-methyl-morpholine;
In
N,N-dimethyl-formamide;
at 20 ℃;
for 24h;
With
trifluoroacetic acid;
In
chloroform;
|
54% |
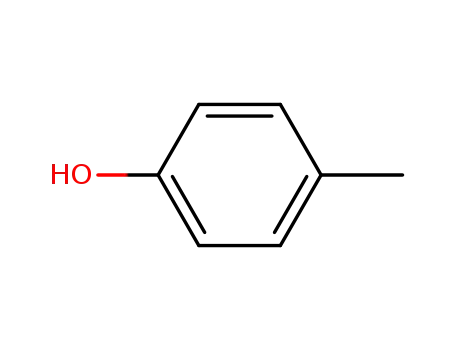
p-cresol

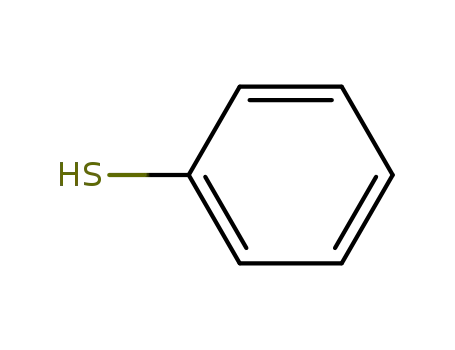
thiophenol


Arg-Gly-Asp
| Conditions | Yield |
|---|---|
|
In
hydrogen fluoride;
|

glycyl-L-aspartic acid

Nα,NG,NG'-tri-Boc-L-arginine N-hydroxysuccinimide ester
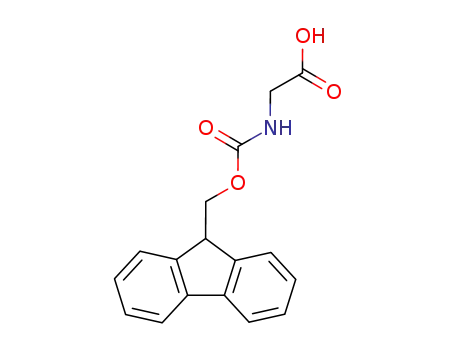
N-(fluoren-9-ylmethoxycarbonyl)glycine
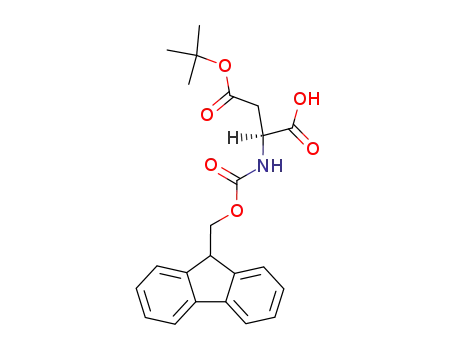
Fmoc-(tBu)Asp-OH
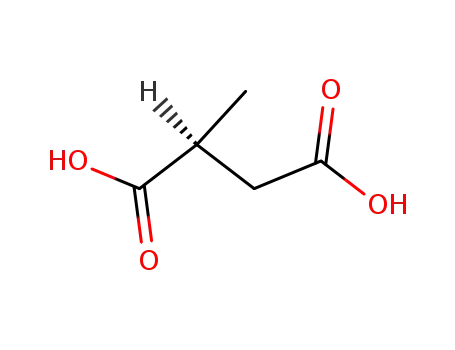
L-malic acid
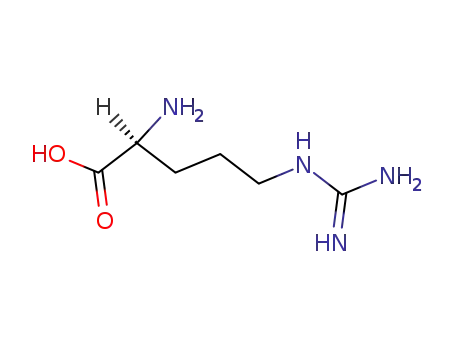
L-arginine
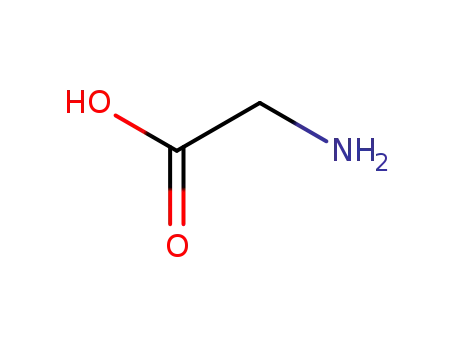
glycine
CAS:119356-77-3
CAS:868844-74-0
CAS:2322-77-2
CAS:10161-34-9