- +86 15383000851
- +86 15303238802
- admin@hebeianda.cn
Your Location:Home >Products >109555-87-5
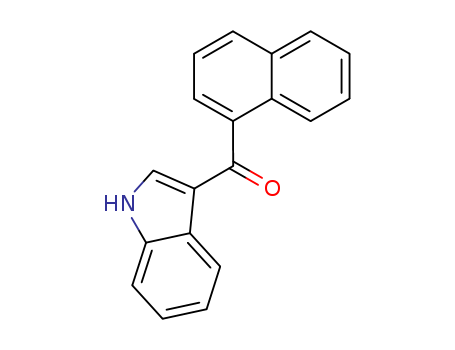

pd_meltingpoint:236 °C
Appearance:Pale pink solid
Purity:99%
|
Chemical Properties |
Pale Pink Solid |
|
Uses |
Metabolite of JWH-018 (P283650). |
InChI:InChI=1/C19H13NO/c21-19(17-12-20-18-11-4-3-9-15(17)18)16-10-5-7-13-6-1-2-8-14(13)16/h1-12,20H
In order to obtain novel pharmacological...
Synthetic cannabinoids are a group of co...
Synthetic cannabinoids (SCs) are a signi...
An efficient regioselective C-3 acylatio...
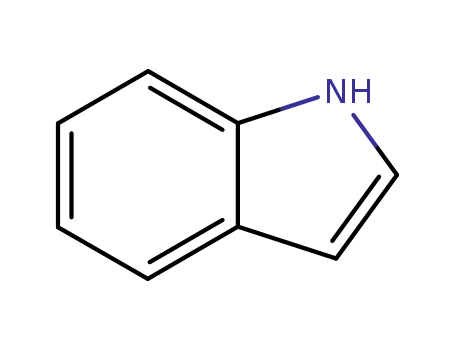
indole

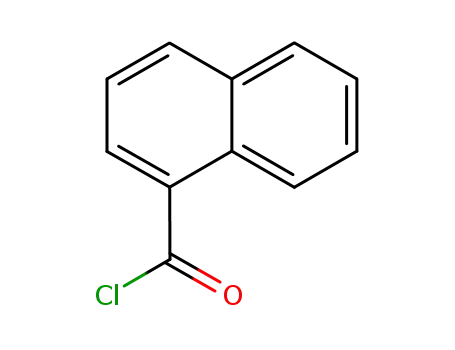
naphthalene-1-carbonic acid chloride

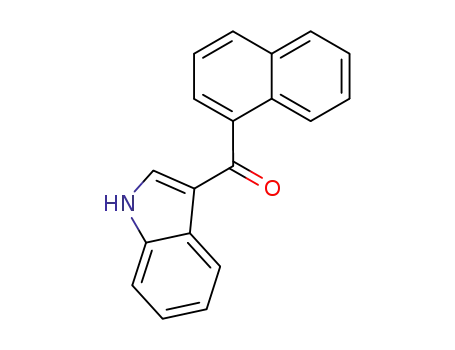
(1H-indol-3-yl)(naphthalene-1-yl)methanone
| Conditions | Yield |
|---|---|
|
With
diethylaluminium chloride;
In
toluene;
at 0 - 20 ℃;
for 24h;
|
93% |
|
indole;
With
methylmagnesium bromide;
In
diethyl ether;
at 20 ℃;
for 2h;
Inert atmosphere;
naphthalene-1-carbonic acid chloride;
In
diethyl ether;
at 0 - 20 ℃;
for 2h;
With
ammonium chloride;
In
diethyl ether;
at 20 ℃;
|
91% |
|
indole;
With
diethylaluminium chloride;
In
dichloromethane; toluene;
at 0 ℃;
for 0.5h;
naphthalene-1-carbonic acid chloride;
In
dichloromethane; toluene;
at 0 ℃;
for 16h;
|
70% |
|
With
zirconium(IV) chloride;
In
1,2-dichloro-ethane;
at 30 ℃;
for 20h;
Inert atmosphere;
|
64% |
|
With
MeMgX;
In
diethyl ether;
|
|
|
With
methylmagnesium bromide; ammonium chloride;
|
|
|
With
aluminum oxide;
In
neat (no solvent);
at 100 ℃;
for 0.133333h;
Microwave irradiation;
Green chemistry;
|
|
|
With
methylmagnesium bromide; ammonium chloride;
|
|
|
indole;
With
ethylmagnesium bromide;
In
diethyl ether;
at 0 - 20 ℃;
for 0.5h;
naphthalene-1-carbonic acid chloride;
In
diethyl ether;
at 20 ℃;
for 1.5h;
|
0.25 g |

indole

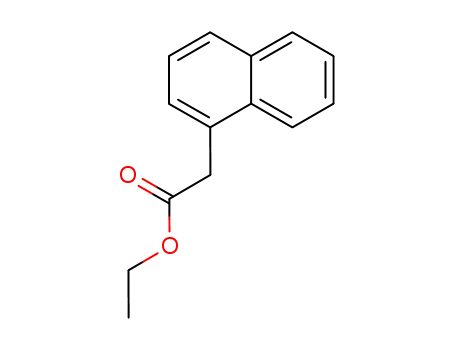
ethyl 2-(1-naphthyl)acetate


(1H-indol-3-yl)(naphthalene-1-yl)methanone
| Conditions | Yield |
|---|---|
|
With
potassium tert-butylate; copper diacetate; dimethyl sulfoxide;
at 110 ℃;
for 24h;
regioselective reaction;
|
67% |

indole
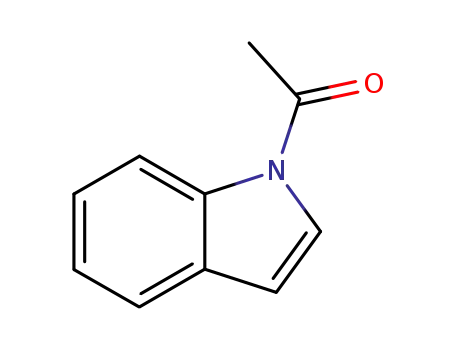
N-acetylindole
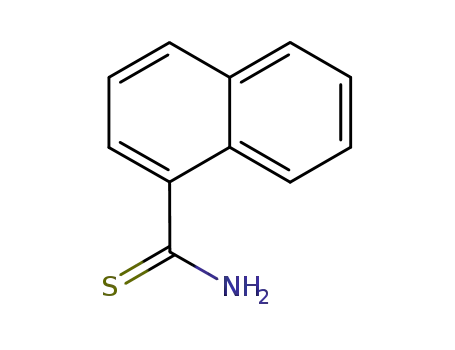
1-naphthalenecarbothioamide
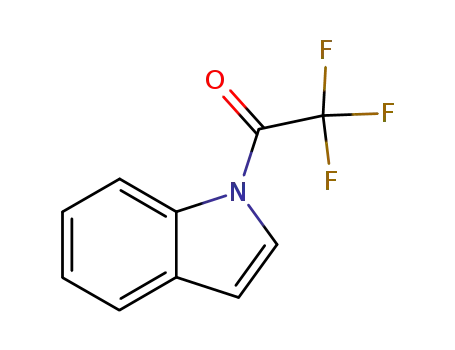
N-(trifluoroacetyl)indole
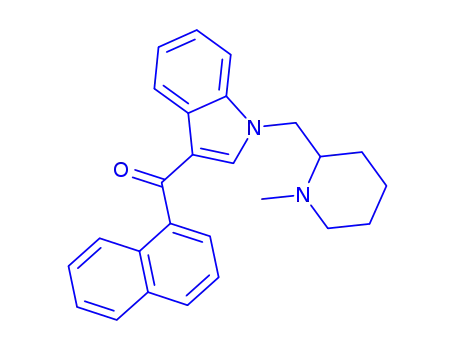
[1-[(1-methyl-2-piperidinyl)methyl]-1H-indol-3-yl]-1-naphthalenylmethanone
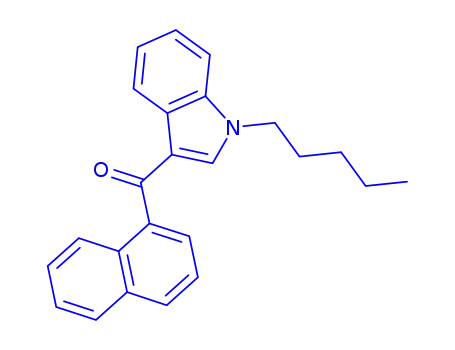
1-pentyl-3-(1-naphthoyl)indole
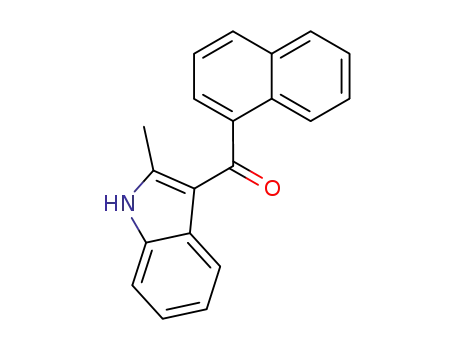
(2-methyl-1H-indol-3-yl)(naphthalen-1-yl) methanone
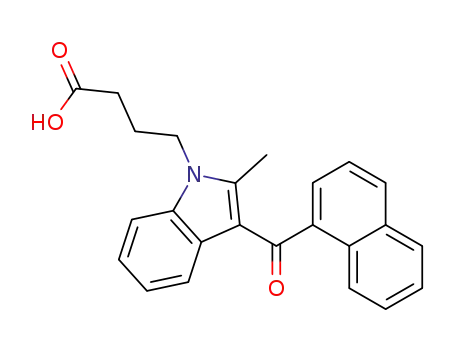
4-(3-(1-naphthoyl)-2-methyl-1H-indol-1-yl)butanoic acid
CAS:119356-77-3
CAS:868844-74-0
CAS:107868-30-4
CAS:69430-36-0