- +86 15383000851
- +86 15303238802
- admin@hebeianda.cn
Your Location:Home >Products >Biochemical Engineering >80714-61-0
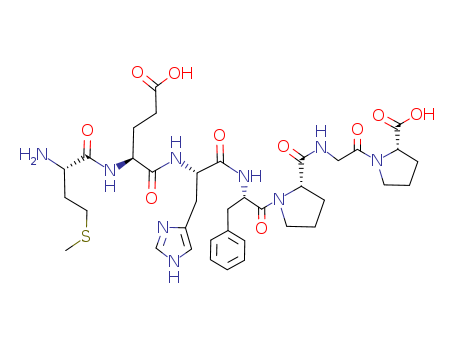

Purity:99%
|
Description |
Semax is a synthetic analogue of the adrenocorticotropic hormone, a hormone responsible for the production of cortisol which in turn regulates glucose and lipid metabolism and helps to maintain blood pressure. In the hippocampus, Semax rapidly elevates the levels of brain-derived neurotrophic factor (BDNF), a protein important in encouraging growth and differentiation of new neurons and synapses. BDNF is active in the hippocampus, cortex, and forebrain and is important for memory, coordination, concentration, and learning. Semax also works to activate the dopaminergic and serotonergic systems to help prime the brain for action and to induce a controlled state of mental stress. |
|
Uses |
Over continued use, guests have seen benefits such as:Reduces anxiety and depression;Improves mental clarity, memory, and focus. Semax has undergone extensive study in Russia and is on the Russian List of Vital & Essential Drugs approved by the Russian Federation government on December 7, 2011. Medical uses for Semax include treatment of stroke, transient ischemic attack, memory and cognitive disorders, peptic ulcers, optic nerve disease, and to boost the immune system. |
|
Mechanism of action |
Semax has been prescribed for anxiety, memory improvement, ischemic events, stroke, nerve regeneration, ADHD, opioid withdrawal and even chronic diseases such as ALS, Parkinson’s Disease, and Alzheimer’s. Semax has been known to be used as an Adderall alternative. |
InChI:InChI=1/C37H51N9O10S/c1-57-16-13-24(38)32(50)42-25(11-12-31(48)49)33(51)43-26(18-23-19-39-21-41-23)34(52)44-27(17-22-7-3-2-4-8-22)36(54)46-15-5-9-28(46)35(53)40-20-30(47)45-14-6-10-29(45)37(55)56/h2-4,7-8,19,21,24-29H,5-6,9-18,20,38H2,1H3,(H,39,41)(H,40,53)(H,42,50)(H,43,51)(H,44,52)(H,48,49)(H,55,56)/t24-,25-,26?,27-,28-,29-/m0/s1
-
A scheme is given for the synthesis of a...
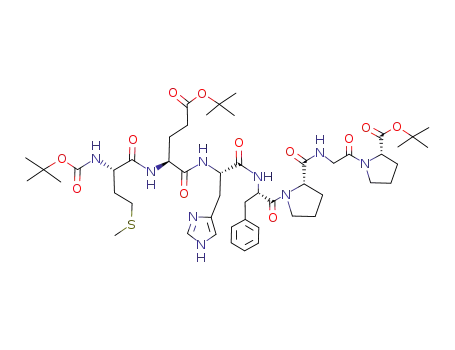
C50H75N9O12S

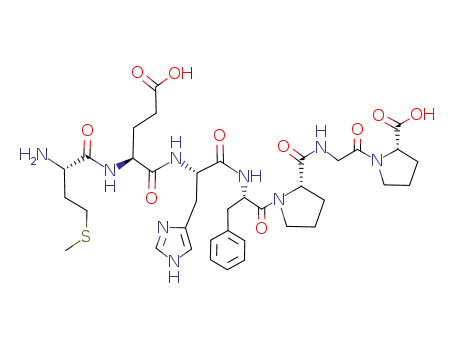
H-Met-Glu-His-Phe-Pro-Gly-Pro
| Conditions | Yield |
|---|---|
|
With dimethylsulfide; trifluoroacetic acid; In various solvent(s); at 30 ℃; for 3h;
|
88% |
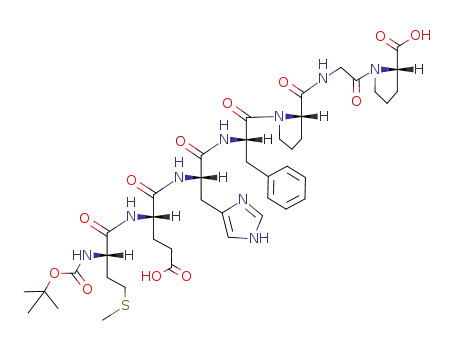
Boc-Met-Glu-His-Phe-Pro-Gly-Pro


H-Met-Glu-His-Phe-Pro-Gly-Pro
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; In acetic acid; for 0.5h; Ambient temperature;
|
57% |

Boc-Met-Glu-His-Phe-Pro-Gly-Pro

C50H75N9O12S
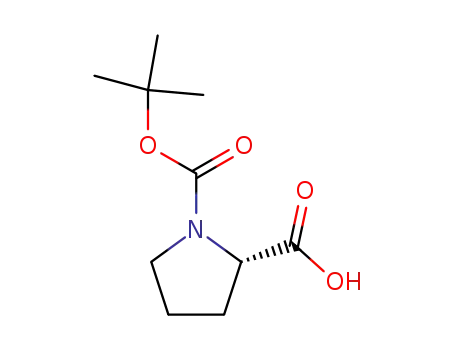
1-(tert-butoxycarbonyl)-L-proline
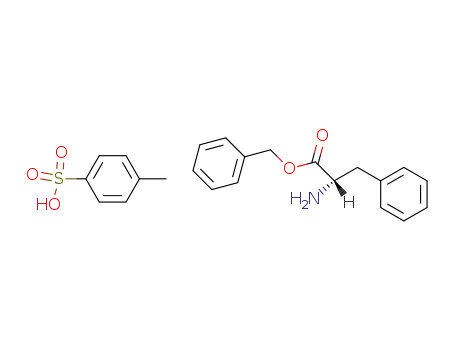
L-phenylalanine benzyl ester p-toluene-sulfonic acid salt
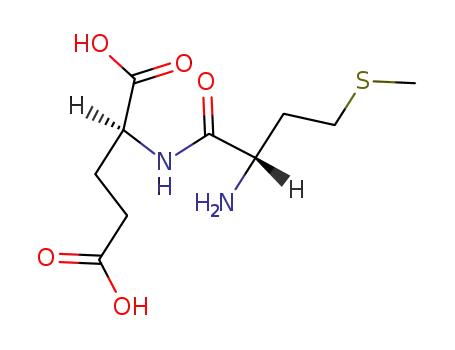
L-methionyl-L-glutamic acid
CAS:868844-74-0
CAS:52-90-4
CAS:70288-86-7
CAS:68521-88-0