- +86 15383000851
- +86 15303238802
- admin@hebeianda.cn
Your Location:Home >Products >Chemical Reagents >22374-89-6


pd_meltingpoint:-50 °C
Appearance:colorless liquid
Purity:99%
|
Chemical Properties |
clear colorless liquid |
|
Uses |
2-Amino-4-phenylbutane is used in the synthesis of N-substituted derivatives of (1-methyl-3- phenylpropyl)amine. |
|
Biological Functions |
Amphetamine is an indirectly acting adrenomimetic amine that depends for its action on the release of norepinephrine from noradrenergic nerves. Its pharmacological effects are similar to those of ephedrine; however, its CNS stimulant activity is somewhat greater. Both systolic and diastolic blood pressures are increased by oral dosing with amphetamine. The heart rate is frequently slowed reflexively. Cardiac output may remain unchanged in the low- and moderate-dose range. The therapeutic uses of amphetamine are based on its ability to stimulate the CNS. The D-isomer (dextroamphetamine) is three to four times as potent as the L-isomer in producing CNS effects. It has been used in the treatment of obesity because of its anorexic effect, although tolerance to this effect develops rapidly. It prevents or overcomes fatigue and has been used as a CNS stimulant. Amphetamine is no longer recommended for these uses because of its potential for abuse. Amphetamine is useful in certain cases of narcolepsy or minimal brain dysfunction. |
|
Flammability and Explosibility |
Notclassified |
|
Safety Profile |
A poison by intraperitoneal and parenteral route. Moderately toxic by ingestion.When heated to decomposition it emits toxic vapors of NOx. |
|
Consumer Uses |
ECHA has no public registered data indicating whether or in which chemical products the substance might be used. ECHA has no public registered data on the routes by which this substance is most likely to be released to the environment. |
InChI:InChI=1/C10H15N/c1-9(11)7-8-10-5-3-2-4-6-10/h2-6,9H,7-8,11H2,1H3/p+1/t9-/m0/s1
A series of half-sandwich Ir(iii) comple...
Half-sandwich iridium complexes bearing ...
Selective introduction of the deuterium ...
Efficient synthesis of primary amines vi...
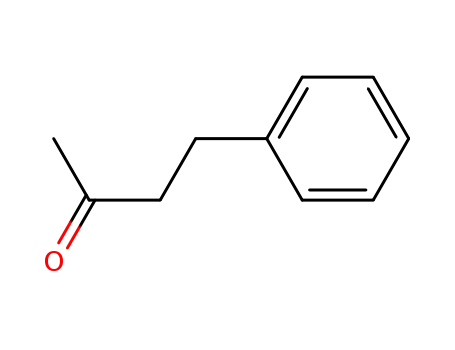
4-Phenyl-2-butanone

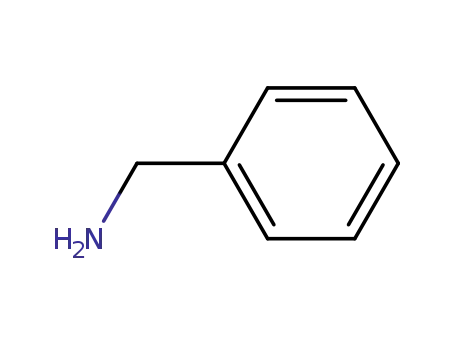
benzylamine


(RS)-1-methyl-3-phenylpropylamine

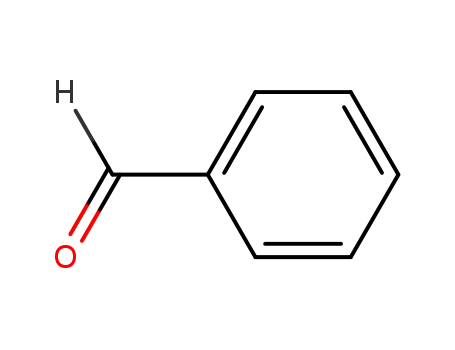
benzaldehyde
| Conditions | Yield |
|---|---|
|
With S-selective ω-transaminase from paracoccus denitrificans transaminase; NAD; aldehyde dehydrogenase; In dimethyl sulfoxide; Reagent/catalyst; Enzymatic reaction;
|
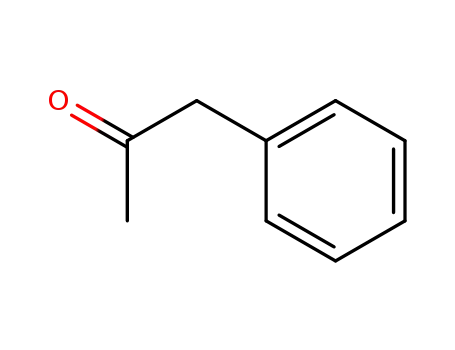
1-phenyl-acetone


(RS)-1-methyl-3-phenylpropylamine

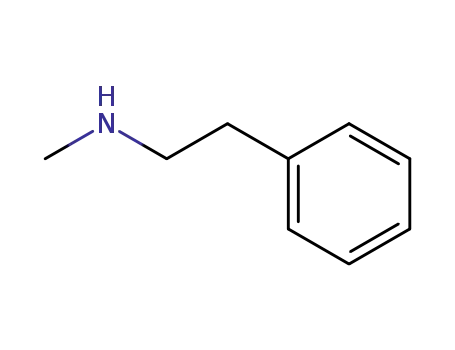
N-Methyl-N-phenethylamine
| Conditions | Yield |
|---|---|
|
With ammonium acetate; magnesium; In methanol; water; at 20 - 25 ℃; Yield given. Yields of byproduct given;
|
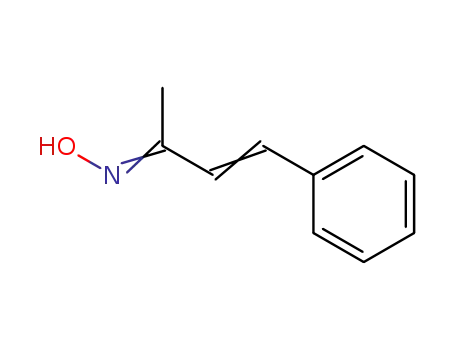
benzalacetone oxime

4-Phenyl-2-butanone
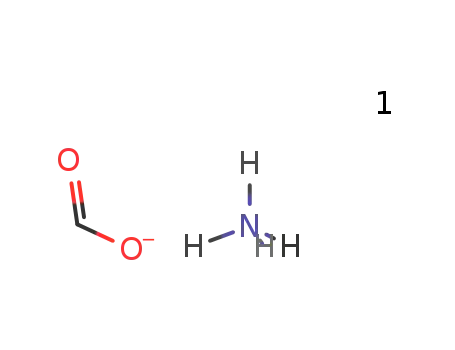
ammonium formate
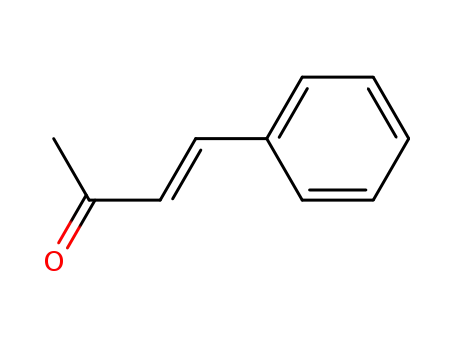
(E)-benzalacetone
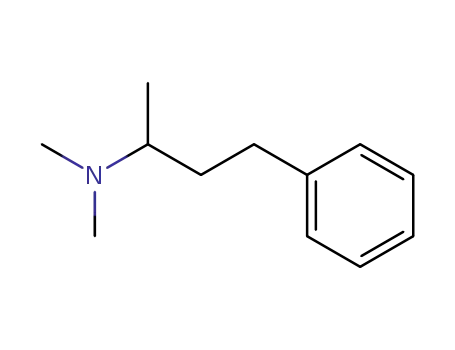
N,N-dimethyl-4-phenyl-2-butanamine
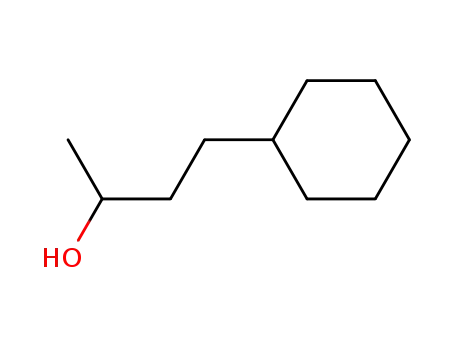
4-cyclohexyl-2-butanol
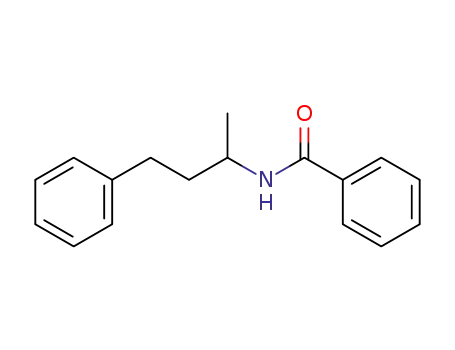
N-(1-phenylbutan-3-yl)benzamide
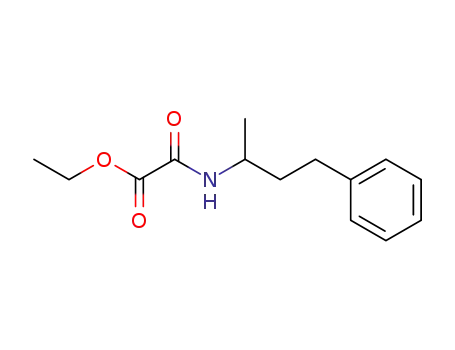
(1-methyl-3-phenyl-propyl)-oxalamic acid ethyl ester
CAS:5197-95-5
CAS:119356-77-3
CAS:1009-14-9
CAS:24305-27-9