- +86 15383000851
- +86 15303238802
- admin@hebeianda.cn
Your Location:Home >Products >Biochemical Engineering >2627-69-2
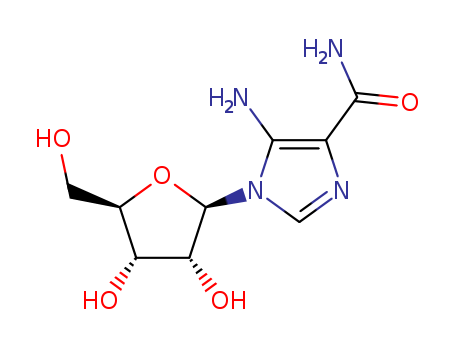

pd_meltingpoint:214-215 °C
Appearance:tan powder
Purity:99%
|
adenosine regulating agents |
Acadesine is the prototype of a new class of compounds termed adenosine regulating agents. Acadesine is a purine nucleosid analogue that enters the myocyte and is immediately phosphorylated to ZMP (AICA ribotide), which is further metabolised to Inosine mono-phosphate (an intermediate in the synthesis ofadenosine triphosphate (ATP) and guanosine triphos-phate). Claims that acadesine may serve as a substrate for ATP synthesis and result in repletion of myocardial ATP were supported by some studies and refuted by others. Because acadesine may be a precursor in the synthesis of myocardial ATP it was proposed as a possible agent of myocardial protection during ischaemia, particularly because myocardial ATP depletion has been linked to cell death. |
|
Description |
AICAR (2627-69-2) activates AMP-activated protein kinase (AMPK). Promotes ligand-independent activation of the insulin receptor.1 Promotes skeletal muscle autophagy via activation of FoxO3a.2 Controls smooth muscle cell hyperproliferation in vascular disease.3 ?Induces osteogenic differentiation in mesenchymal stem cells.4 Inhibits proinflammatory response in glial cells.5 Cell permeable. |
|
Chemical Properties |
Solid |
|
Originator |
AICA,BIOMOL |
|
Uses |
glucose uptake stimulant; AMPK activator |
|
Definition |
ChEBI: A 1-ribosylimidazolecarboxamide in which the carboxamide group is situated at position 4 of the imidazole ring, which is further substituted at position 5 by an amino group. A purine nucleoside analogue and activator of AMP-activated protein kinase, it is is used for the treatment of acute lymphoblastic leukemia and is reported to have cardioprotective effects. |
|
Manufacturing Process |
Adenosine 3', 5'-cyclic phosphate N'-oxide (76.0 g, 0.200 mole) as the dihydrate was dissolved in a solution of 400 ml DMSO and 31.0 g (0.204 mole) 1,5-diazabicyclo[5.4.0]undec-5-ene. The solution was cooled to 15°C and 40 ml methyl iodide was added with stirring at room temperature. After 30 min, the mixture had gelled; 1.5 L ethanol was added and the solid was thoroughly homogenized by vigorous stirring. The solid was filtered, and the resulting paste was resuspended in 2 L ethanol and homogenized. The product was again filtered, washed with ethanol and ether, and dried, giving 80.4 g of 1-methoxyadenosine 3',5'-cyclic phosphate suitable for further transformation (recrystallization from aqueous methanol with ether). A solution of 30.0 g 1-methoxyadenosine 3',5'-cyclic phosphate (81.5 mmole), 20.0 g NaHCO3 (238 mmole), and 300 ml H2O was refluxed 45 mm. The pH of the solution was adjusted to 2.5 with Dowex 50x8 (H)+ while warm, and a water pump vacuum was applied to mixture to remove CO2. The pH was readjusted to 9-10 with NaOH, and the resin was removed by filtration. The solution was passed onto a column containing 400 ml Dowex 1x2 (formate, 100-200 mesh), and the column was washed well with water. The column was eluted with a gradient of 4 L water in the mixing chamber and 4 L 4 N formic acid in the reservoir. The first major product, coming after about 2 L eluate, was 5-amino-N-methoxy-1-β-D-ribofuranosylimidazole-4-carboxamidine 3',5'- cyclic phosphate, giving 5.4 g (19%) after evaporation of the solvent and trituration of the residue with ethanol (recrystallization from water). A solution of 5.0 g (14.3 mmoles) 5-amino-N-methoxy-1-β-Dribofuranosylimidazole- 4-carboxamidine 3',5'-cyclic phosphate in 200 ml H2 preheated to 60°C and containing approximately 5.0 g moist sponge nickel catalyst, was shaken with 2-3 atm. H2 at 60°C for 2 h. The filtered solution was evaporated to dryness to give 3.75 g of 5-amino-1-β-Dribofuranosylimidazole- 4-carboxamidine 3',5'-cyclic phosphate (82%), (recrystallization from water). A mixture of 4.0 g (12.5 mmole) 5-amino-1-β-D-ribofuranosylimidazole-4- carboxamidine 3',5'-cyclic phosphate and 100 ml conc. NH4OH was heated in a bomb at 100°C for 16 h, then cooled and evaporated in vacuum. The residue was taken up in 100 ml H2O and applied to a 2.5x20 cm column of Dowex 1x2 (formate form, 100-200 mesh). After washing well with H2O the column was eluted with a gradient of 1 L H2O in the mixing chamber and 1 L 3 N formic acid in the reservoir. Fractions containing the product, appearing near the end of the elution, were evaporated. Trituration of the residue with EtOH gave 2.90 g (68%) of 5-amino-1-β-D-ribofuranosylimidazole-4- carboxamide 3',5'-cyclic phosphate. The 5-amino-1-β-D-ribofuranosylimidazole-4-carboxamide may be produced by hydrolysis of 5-amino-1-β-D-ribofuranosylimidazole-4-carboxamide 3',5'- cyclic phosphate with NaOH. |
|
Therapeutic Function |
Cardiotonic, Platelet aggregation inhibitor |
|
General Description |
Adenosine monophosphate?protein kinase (AMPK) activator 5-aminoimidazole-4-carboxamide?ribonucleotide?(AICAR)?modulates cellular energy. It regulates lipid and glucose metabolism, pro-inflammatory responses,?cytokine production,?cell proliferation and apoptosis. AICAR improves ischemia or reperfusion injury and kidney fibrosis in rats. It provides protection against acute tubular necrosis. |
|
Biological Activity |
Cell-permeable, allosteric activator of AMP-activated protein kinase (AMPK). Augments proliferation, differentiation and mineralization of osteoblastic MC3T3-EI cells and attenuates psychosine-induced expression of proinflammatory cytokines and iNOS in astrocytes. |
|
Biochem/physiol Actions |
AICAR is a cell permeable activator of AMP-activated protein kinase (AMPK), a metabolic master regulator that is activated in times of reduced energy availability (high cellular AMP:ATP ratios) and serves to inhibit anabolic processes. In vivo, pharmacologic activation of AMPK with AICAR mimics exercise and triggers insulin-independent glucose uptake by skeletal muscle. |
InChI:InChI=1/C9H14N4O5/c10-7-4(8(11)17)12-2-13(7)9-6(16)5(15)3(1-14)18-9/h2-3,5-6,9,14-16H,1,10H2,(H2,11,17)/t3-,5-,6-,9-/m1/s1
The enzyme aminoimidazole ribonucleotide...
The synthesis and the use of new N-1-din...
Herein, we report the synthesis and the ...
The invention relates to analogous compo...
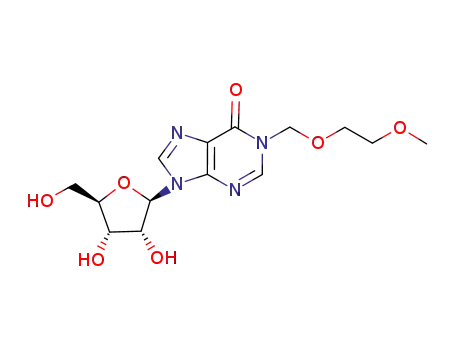
9-((2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-1-((2-methoxyethoxy)methyl)-1,9-dihydro-6H-purin-6-one

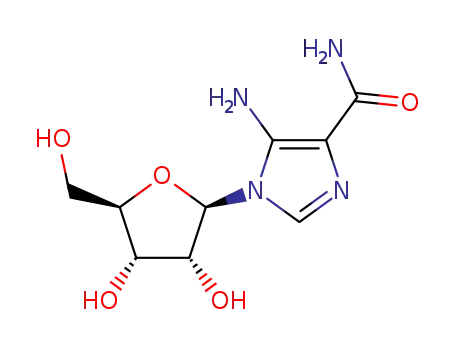
AICA riboside
| Conditions | Yield |
|---|---|
|
With sodium hydroxide; for 1h; Reflux;
|
74% |
|
With sodium hydroxide; for 1h; Heating;
|
73% |
|
9-((2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-1-((2-methoxyethoxy)methyl)-1,9-dihydro-6H-purin-6-one; With sodium hydroxide; water; for 1h; Heating / reflux;
With hydrogenchloride; In water; at 20 ℃;
|
70% |
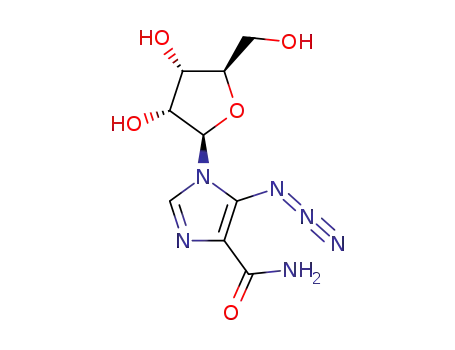
5-azido-1-β-D-ribofuranosyl-1H-imidazole-4-carboxamide


AICA riboside
| Conditions | Yield |
|---|---|
|
With hydrogen; palladium on activated charcoal; In acetic acid; for 1.5h; under 760 Torr; Ambient temperature;
|
42% |
|
With hydrogen; palladium on activated charcoal; In water; acetic acid; at 23 ℃; for 1h; under 760 Torr;
|
42% |
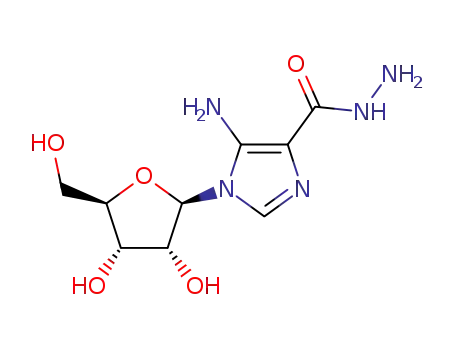
5-Amino-1-(β-D-ribofuranosyl)imidazole-4-carboxylic Acid Hydrazide
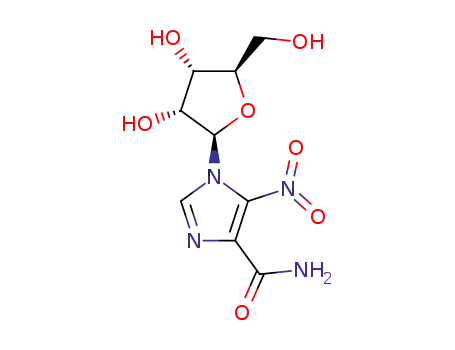
5-nitro-1-β-D-ribofuranosyl-1H-imidazole-4-carboxylic acid amide
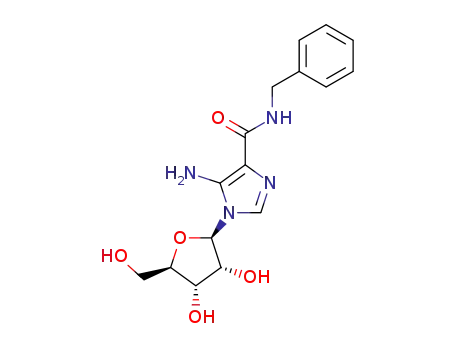
5-amino-1-(β-D-ribofuranosyl)imidazole-4-(N-benzyl)carboxamide
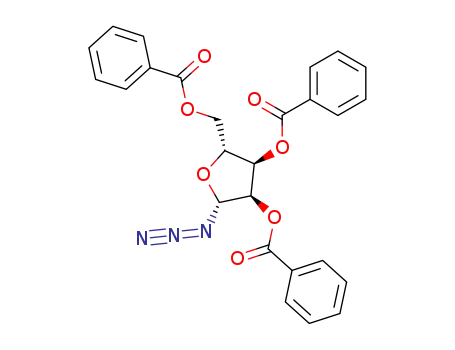
2,3,5-tri-O-benzoyl-β-D-ribofuranosyl azide
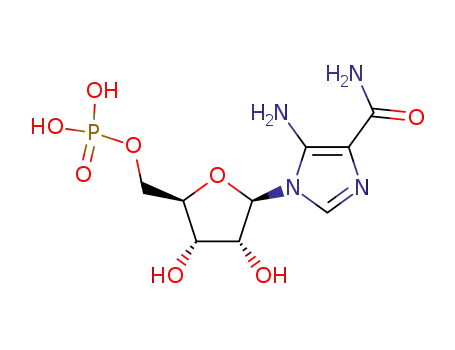
5-aminoimidazole-4-carboxamide ribonucleotide
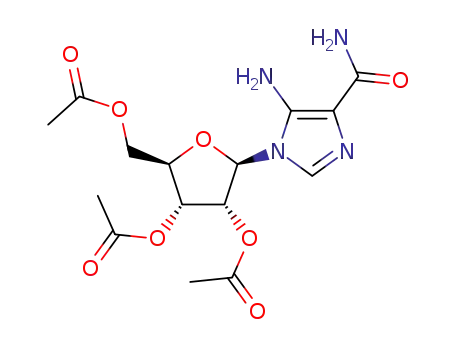
5-Amino-4-carbamoyl-1β-(2',3',5'-tri-O-acetyl-D-ribofuranosyl)imidazole
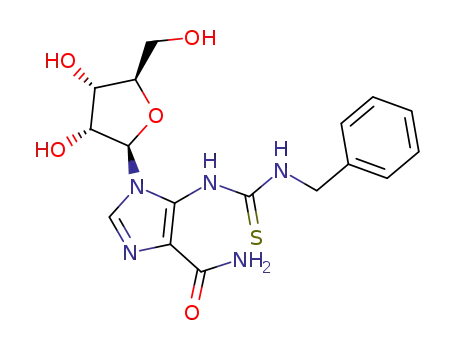
5-<1-(3-benzylthioureido)>-1-(β-D-ribofuranosyl)imidazole-4-carboxamide
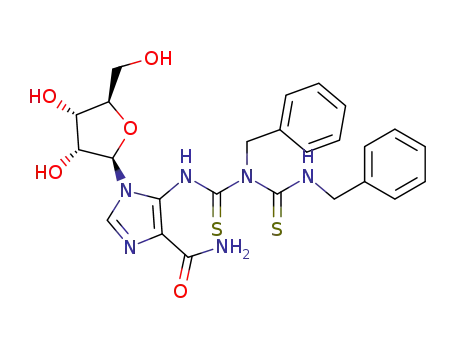
5-<1-<3-(benzylthiocarbamoyl)-3-benzylthioureido>>-1-(β-D-ribofuranosyl)imidazole-4-carboxamide
CAS:119356-77-3
CAS:868844-74-0
CAS:137525-51-0