- +86 15383000851
- +86 15303238802
- admin@hebeianda.cn
Your Location:Home >Products >API >144701-48-4
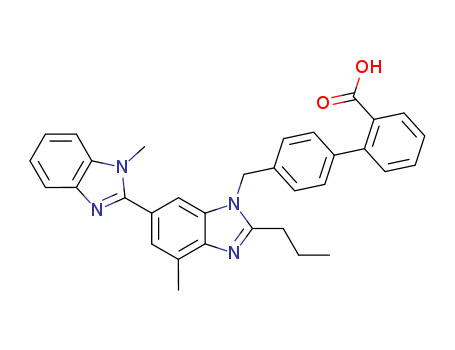

pd_meltingpoint:261-263 °C
Appearance:White or off white crystalline powder
Purity:99%
|
Chemical Properties |
White or off-white crystalline powder, odorless and tasteless. Soluble in chloroform, slightly soluble in methanol, very slightly soluble in acetone, practically insoluble in water. Slightly soluble in 0.1mol/L hydrochloric acid, soluble in 0.1mol/L sodium hydroxide. Absorption coefficient(E1%1cm):509~541 |
|
Mechanisms of Action |
80mg of telmisartan for the human body will practically completely counter the high blood pressure caused by angiotension II. Effects will last for 24 and can still be detected within 48 hours. Blood pressure-lowering effects should gradually become apparent within 3 hours after the initial dose. If treatment is suddenly interrupted, blood pressure will return to the same level as before treatment in a matter of days, and there will be no rebound high blood pressure. In a clinical trial that compared two kinds of antihypertensive drugs, patients in the treatment group experienced lower rates of coughing than those in the group treated by angiotensin converting enzyme inhibitors. Telmisartan has a half-life of 18~24 hours, and will take effect 1~4 hours after taken. Medicinal effects can last for as long as 35 hours, with a high (T/P) ratio and outstanding effects in controlling morning blood pressure. Thus, this medicine can effectively control blood pressure for 24 hours, and it meets the once-daily medicinal standard (40~80mg, qd). Clinical effects and characteristics: Pharmacokinetics show: Rapid effects (0.3h), long duration (35.4h), little effect on heart rate when lowering blood pressure. Compared to Enalapril: Stronger antihypertensive effects than Enalapril; when both are used in combination with diuretics, Telmisartan still appears to be superior and results in a lower coughing rate. Compared to Lisinopril: More apparent antihypertensive effects (for both systolic and diastolic blood pressure), coughing rate for Telmisartan (16%) is drastically lower than that of Lisinopril (60%). Compared to Atenolol: Similar antihypertensive effects, lower rate of side effects (impotence and fatigue). Compared to Amlodipine: The Telmisartan group experienced noticeably lower heart rates four hours after ingestion and from six a.m. to twelve p.m. Overall, Telmisartan has the following characteristics in comparison with other antihypertensive medicine: specific receptor effects, noticeable hypertensive effects, good diuretic effects, improvement of myocardial stenosis, safe usage, good tolerance, and convenient daily dosage. |
|
Side Effects |
In the placebo control experiment, the incidence rate of negative events for the Telmisartan group (41.4%) was similar to that of the placebo group (43.9%). Negative events were unrelated to dosage, sex, age, or race. The following lists negative reactions were accumulated via the 5788 hypertensive patients that were treated with Telmisartan in the clinical trial. Negative effects are categorized by incident rate as: Very common (>1/10); common (>1/100, <1/10=); uncommon (>1/1000, <1/100=); rare (>1/10000, <1/1000=); very rare (<1/100000=) Bodily reaction: Common: Back pain (e.g. sciatica), chest pain, flu-like symptoms, infection symptoms (e.g. urinary tract infection including cystitis). Uncommon: sight abnormality, sweating. Central and peripheral nervous system: Common: vertigo. Digestive system: Common: stomach pain, diarrhea, indigestion, gastrointestinal disorders. Rare: dry mouth, bloating. Muscle skeletal system: Common: joint pain, leg cramps or leg pain, muscle pain. Rare: Tenosensitis symptoms. Nervous system: Rare: anxiety. Respiratory system: Common: upper respiratory tract infection, including pharyngitis and rhinitis. Skin and accessory system: Common: Skin abnormalities such as eczema. Additionally, since Telmisartan entered the market, there have been individual cases of erythema, itching, syncope, insomnia, depression, stomach discomfort, vomiting, hypotension, bradycardia, tachycardia, dyspnea, eosinophilia, thrombocytopenia, weakness, and reduced work efficiency. Similar to other angiotensin II receptor blockers, very few cases have reported vascular edema, nettles, and other negative reactions. The laboratory found: compared to the placebo, the Telmisartan group occasionally exhibited lowered hemoglobin or raised uric acid. Any increase in serum creatinine or liver enzymes in the Telmisartan group was similar or lower than that of the placebo group. |
|
Patent |
Telmisartan was originally formulated by the German pharmaceutical company Boehringer Ingelheim; it earned the German patent EP502,314 in 1991, was first approved to enter the American market in November 1998, and then entered German, Philippines, Australian, Belgium, British, and other markets. |
|
Description |
Telmisartan was launched in the US for the treatment of hypertension. It can be prepared in eight steps starting with methyl 4-amino-3-methyl benzoate; the first and second cyclization into a benzimidazole ring occur at steps 4 and 6 respectively. Telmisartan blocks the action of angiotensin II (Ang II), the primary effector molecule of the renin-angiotensin-aldosterone system (RAAS). It is the sixth of this class of 《sartans》 to be marketed after the lead compound Losartan. Its long lasting effect (24h half-life) could be the main difference with other angiotensin II antagonists. Unlike several other agents in this category, its activity does not depend upon transformation into an active metabolite, the 1-O-acylglucuronide being the principal metabolite found in humans. Telmisartan is a potent competitive antagonist of AT1 receptors that mediate most of the important effects of angiotensin II while lacking affinity for the AT2 subtypes or other receptors involved in cardiovascular regulation. In several clinical studies, Telmisartan, at a once daily dosage, produced effective and sustained blood-pressure lowering effects with a low incidence of side effects (particularly treatment-related cough associated with ACE inhibitors in elderly patients). |
|
Originator |
Boehringer Ingelheim (Germany) |
|
Uses |
cardiotonic |
|
Definition |
ChEBI: A member of the class of benzimidazoles used widely in the treatment of hypertension. |
|
Manufacturing Process |
A solution of 23.9 g (100 mMol) of methyl 3,4-diaminobenzoate dihydrochloride and 11.7 g (110 mMol) of butyric acid chloride in 100 ml of phosphorus oxychloride is refluxed for 2 h. Then about 80 ml of phosphorus oxychloride are distilled off and the residue is mixed with about 150 ml of water. The oily crude product precipitated is extracted three times with 50 ml of ethyl acetate and after evaporation purified by column chromatography (600 g of silica gel; eluant:methylene chloride/methanol (30:1)). Yield of methyl-2-n-propyl-benzimidazole-5-carboxylate: 15.0 g of oil (69%).A solution of 15.0 g (73 mmol) of methyl 2-n-propyl-benzimidazole-5- carboxylate and 8 g (200 mMol) of sodium hydroxide in 200 ml of water and 400 ml of ethanol is refluxed for 2 h. Then the alcohol is distilled off, the aqueous solution is acidified with dilute sulphuric acid (pH 4-5) and evaporated using a rotary evaporator. The product crystallising out is suction filtered, washed with 50 ml of acetone and 50 ml of diethylether and dried. Yield of 2-n-propyl-benzimidazole-5-carboxylic acid-hemisulphate: 9.1 g (61%), melting point: >220°C.A solution of 6.7 g (25 mMol) of 2-n-propyl-benzimidazole-5-carboxylic acidhemisulphate and 4.9 g (25 mMol) of 2-methylaminoaniline dihydrochloride in 200 g of polyphosphoric acid is stirred for 5 h at 150°C, then poured onto 600 ml of water and made alkaline with concentrated ammonia whilst cooling with ice. The resulting solution is extracted three times with 200 ml of ethyl acetate, the crude product thus obtained is purified by column chromatography (300 g of silica gel; eluant:methylene chloride/methanol = 15:1). Yield of 2-n-propy1-5-(1-methylbenzimidazol-2-yl)-benzimidazole: 2.8 g of oil (39%).A solution of 2.0 g (6.9 mMol) of 2-n-propyl-5-(1-methylbenzimidazol-2-yl)- benzimidazole and 0.91 g (7.5 mmol) of potassium tert-butoxide in 50 ml of dimethylsulfoxide is stirred for 90 min at room temperature, then 2.6 g (7.5 mMol) of tert-butyl 4'-bromomethyl-biphenyl-2-carboxylate are added and the mixture is stirred for a further 15 h at room temperature. The mixture is then poured onto 300 ml of water and extracted three times with 50 ml of ethyl acetate. The crude product obtained after evaporation of the organic phase is purified by column chromatography (300 g silica gel; eluant:methylene chloride/methanol = 30:1). In this way, 2.7 g (70%) of an isomer mixture are obtained (by NMR spectroscopy), contains about 1.18 g of tert-butyl-4'-[(2-npropyl- 5-(1-methylbenzimidazol-2-yl)-benzimidazol-1-yl)-methyl]biphenyl-2- carboxylate and about 1.52 g of tert-butyl 4'-[(2-n-propyl-6-(1- methylbenzimidazol-2-yl)-benzimidazol-1-yl)-methyl]biphenyl-2-carboxylate).2.70 g of the isomer mixture obtained above are dissolved in 100 ml of methylene chloride, mixed with 40 ml of trifluoroacetic acid and stirred for 4 h at room temperature. The mixture is then evaporated to dryness in vacuo, the residue is dissolved in 100 ml of 2 N sodium hydroxide solution, the solution is washed with 50 ml of diethylether and the product mixture is precipitated by acidifying the aqueous phase with acetic acid. By column chromatography (400 g of silica gel, eluant:methylene chloride/methanol = 15:1) of the solid thus obtained 0.9 g (74%) of 4'-[[2-n-propyl-6-(1-methylbenzimidazol-2-yl)- benzimidazol-1-yl]methyl]biphenyl-2-carboxylate, melting point 217°-218°C. |
|
Brand name |
Micardis (Boehringer Ingelheim). |
|
Therapeutic Function |
Antihypertensive |
|
General Description |
Telmisartan, 4'-[(1,4'-dimethyl-2'-propyl[2,6'-bi-1H-benzimidazol]-1'-yl)methyl]-[1,1'-biphenyl]-2-carboxylic acid (Micardis), does not appear to bear any structuralrelationship to this class, but there is actually a great dealof overlap in the chemical architecture with other agents. Thefirst, and most significant, difference is the replacement of theacidic tetrazole system with a simple carboxylic acid. Thisacid, like the tetrazole, plays a role in receptor binding. Thesecond difference is the lack of a carboxylic acid near the imidazolenitrogen that also contributes to receptor binding.As with irbesartan, however, there is not a need for this groupto be acidic but, rather, to be one that participates in receptorbinding. The second imidazole ring, much like a purine basein deoxyribonucleic acid (DNA), can hydrogen bond with theangiotensin II receptor. |
|
Biochem/physiol Actions |
Telmisartan is a non-peptide AT1 angiotensin receptor antagonist. |
|
Clinical Use |
Angiotensin-II antagonist: Hypertension Prevention of cardiovascular events |
|
Drug interactions |
Potentially hazardous interactions with other drugs Anaesthetics: enhanced hypotensive effect. Analgesics: antagonism of hypotensive effect and increased risk of renal impairment with NSAIDs; hyperkalaemia with ketorolac and other NSAIDs. Antihypertensives: increased risk of hyperkalaemia, hypotension and renal impairment with ACE-Is and aliskiren. Cardiac glycosides: concentration of digoxin increased. Ciclosporin: increased risk of hyperkalaemia and nephrotoxicity. Diuretics: enhanced hypotensive effect; hyperkalaemia with potassium-sparing diuretics. ESAs: increased risk of hyperkalaemia; antagonism of hypotensive effect. Lithium: reduced excretion (possibility of enhanced lithium toxicity). Potassium salts: increased risk of hyperkalaemia. Tacrolimus: increased risk of hyperkalaemia and nephrotoxicity. |
|
Metabolism |
Telmisartan is metabolised by conjugation to the glucuronide of the parent compound. No pharmacological activity has been shown for the conjugate. Telmisartan is excreted almost entirely in the faeces via bile, mainly as unchanged drug. |
InChI:InChI=1/C33H30N4O2/c1-7-12-33-39-34-24(2)21-27(35-38-30-15-10-11-16-31(30)40(35)6)22-32(34)41(33)23-25-17-19-26(20-18-25)28-13-8-9-14-29(28)36(42)43-37(3,4)5/h8-11,13-22H,7,12,23H2,1-6H3
Cost-effective and improved one-pot synt...
A concise synthetic route was designed f...
A direct and efficient total synthesis h...
The invention relates to a preparation m...
PROBLEM TO BE SOLVED: To provide a manuf...
The solid dispersions comprising telmisa...
![tert-butyl 4'-[[2-n-propyl-4-methyl-6-(1-methylbenzimidazol-2-yl)-benzimidazol-1-yl]-methyl]-biphenyl-2-carboxylate](/upload/2023/8/943c8759-cfcf-44b6-acbe-64a2c0919e81.png)
tert-butyl 4'-[[2-n-propyl-4-methyl-6-(1-methylbenzimidazol-2-yl)-benzimidazol-1-yl]-methyl]-biphenyl-2-carboxylate

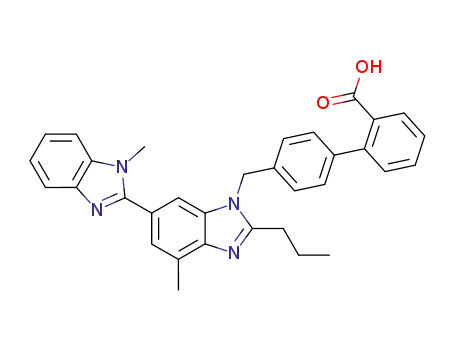
telmisatran
| Conditions | Yield |
|---|---|
|
With
sodium hydroxide;
In
tetrahydrofuran;
at 100 ℃;
Reagent/catalyst;
Solvent;
|
93% |
|
With
trifluoroacetic acid;
In
N,N-dimethyl-formamide;
for 4h;
|
70% |
|
With
trifluoroacetic acid;
In
N-methyl-acetamide;
|
63.9% |
|
With
trifluoroacetic acid;
In
dichloromethane;
for 24h;
Yield given;
Ambient temperature;
|
![tert-butyl-4'-[[2-n-propyl-4-methyl-6-(1-methylbenzimidazol-2-yl)-benzimidazol-1-yl]-methyl]-biphenyl-2-carboxylate hydrochloride](/upload/2023/8/0be63d3d-0ce4-4a8c-95af-08fa913f6e3d.png)
tert-butyl-4'-[[2-n-propyl-4-methyl-6-(1-methylbenzimidazol-2-yl)-benzimidazol-1-yl]-methyl]-biphenyl-2-carboxylate hydrochloride


telmisatran
| Conditions | Yield |
|---|---|
|
tert-butyl-4'-[[2-n-propyl-4-methyl-6-(1-methylbenzimidazol-2-yl)-benzimidazol-1-yl]-methyl]-biphenyl-2-carboxylate hydrochloride;
With
acetic acid;
In
water;
Heating / reflux;
With
sodium hydroxide;
In
water;
at 80 - 90 ℃;
|
93.3% |
|
With
sodium hydroxide; water;
In
water; acetic acid;
at 80 - 90 ℃;
Heating / reflux;
|
93.3% |
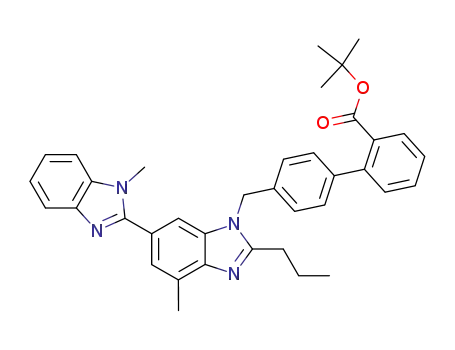
tert-butyl 4'-[[2-n-propyl-4-methyl-6-(1-methylbenzimidazol-2-yl)-benzimidazol-1-yl]-methyl]-biphenyl-2-carboxylate
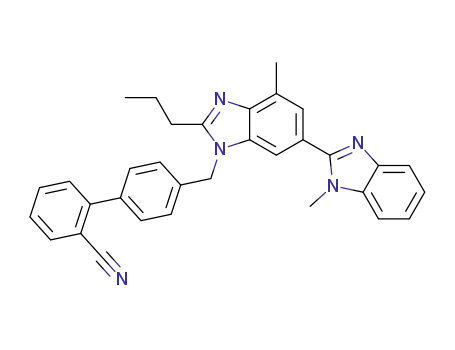
4'-[[2-n-propyl-4-methyl-6-(1-methyl-benzimidazol-2-yl)-1H-benzimidazol-1-yl]-methyl]-2-cyano-biphenyl
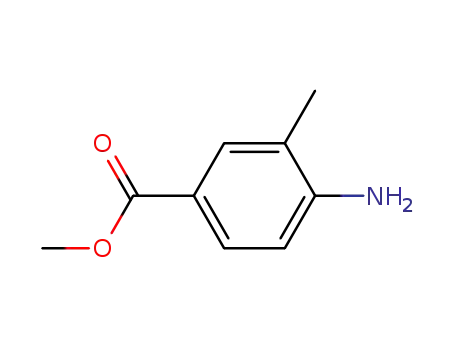
methyl 4-amino-3-methylbenzoate
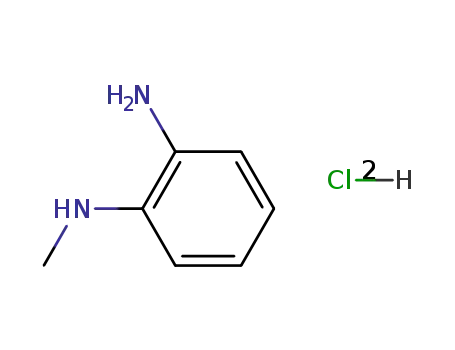
N-methylbenzene-1,2-diamine dihydrochloride
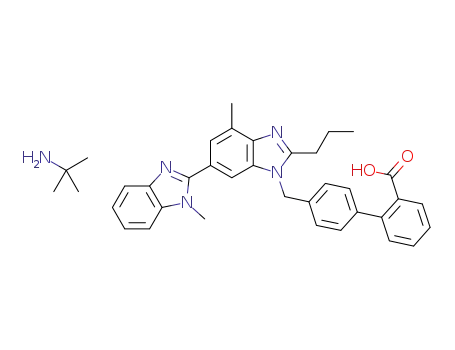
telmisartan tert-butylamine salt
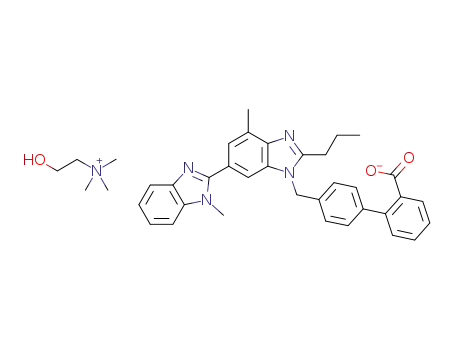
telmisartan choline salt
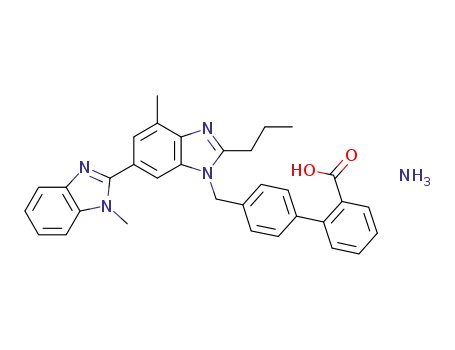
4'-[[2-n-propyl-4-methyl-6-(1-methylbenzimidazol-2-yl)-benzimidazol-1-yl]-methyl]-biphenyl-2-carboxylic acid ammonium salt
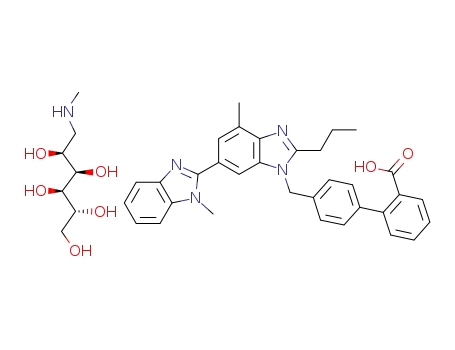
telmisartan meglumine salt
CAS:119356-77-3
CAS:868844-74-0
CAS:55268-74-1
CAS:5197-95-5