- +86 15383000851
- +86 15303238802
- admin@hebeianda.cn
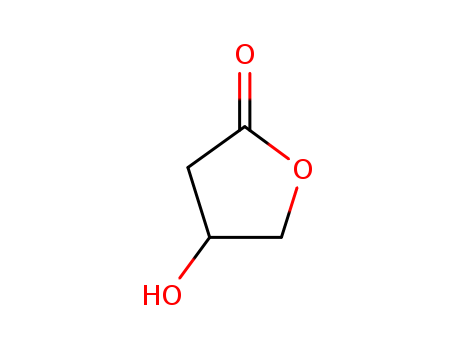

pd_meltingpoint:1.24ºC
Appearance:Colorless to light yellow liquid
Purity:99%
|
Description |
(S)-3-Hydroxy-gamma-butyrolactone is an important intermediate in organic synthesis and an important chiral pool. It is mainly used in the synthesis of some natural products and some bioactive chiral drugs or antibiotic chiral drugs. For example, it is a key intermediate in synthesis of nerve regulator (R)-GABOB and brain metabolic accelerant S-oxiracetam (S-ORC). It can be deoxidized to (S)-(+)-3-Hydroxytetrahydrofuran which is an important intermediate of anti-AIDS drugs. It is also used in the synthesis of S(-)-3-hydroxy-4-bromobutyric acid which is a potential stabilizer. |
|
Chemical Properties |
Colorless to light yellow liquid. |
|
Uses |
(S)-3-Hydroxy-γ-butyrolactone can be used as an anticancer drug resistance inhibitor. |
|
Precautions |
For best results, Store in cool, dry place in tightly closed containers, under inert gas and protected from moisture as this substance is moisture sensitive. (S)-3-Hydroxy-gamma-butyrolactone is incompatible with oxidizing agents. This chemical causes skin irritation and serious eye irritation. |
InChI:InChI=1/C4H6O3/c5-3-1-4(6)7-2-3/h3,5H,1-2H2/t3-/m0/s1
Catalytic selective hydrogenation of est...
Kinsenoside is the major bioactive compo...
A concise, stereoselective and protectin...
The invention discloses a synthetic meth...
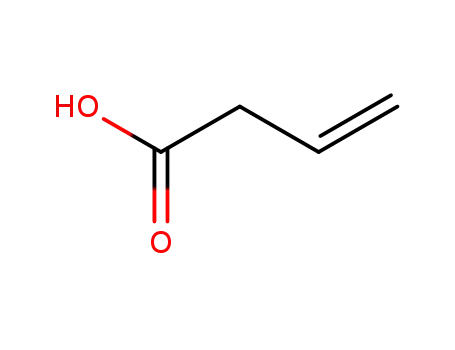
but-3-enoic acid

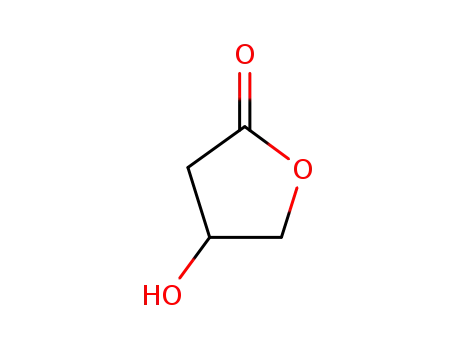
4-Hydroxy-dihydro-furan-2-on
| Conditions | Yield |
|---|---|
|
With
dihydrogen peroxide;
In
tert-butyl alcohol;
|
91% |
|
With
osmium(VIII) oxide; barium chlorate; water;
|
|
|
With
Perbenzoic acid; chloroform;
|
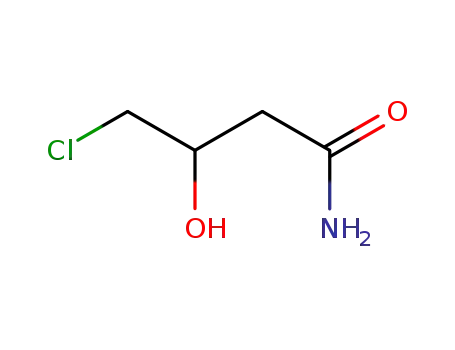
4-chloro-3-hydroxybutylamide


4-Hydroxy-dihydro-furan-2-on
| Conditions | Yield |
|---|---|
|
In
water; butanone;
at 60 ℃;
for 24h;
|
92% |
|
In
water;
at 70 ℃;
for 3h;
|
65.2% |
|
In
water;
at 30 - 100 ℃;
for 0.5 - 336h;
pH=1.2 - 5.5;
Aqueous phophate buffer;
|
56.5% |

but-3-enoic acid
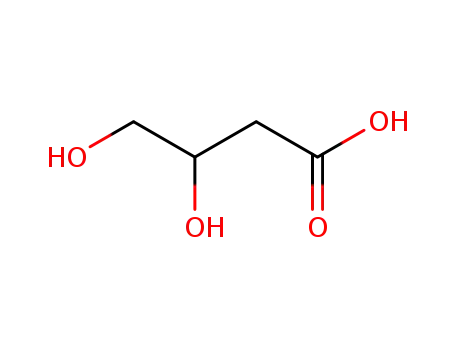
3,4-dihydroxybutyric acid
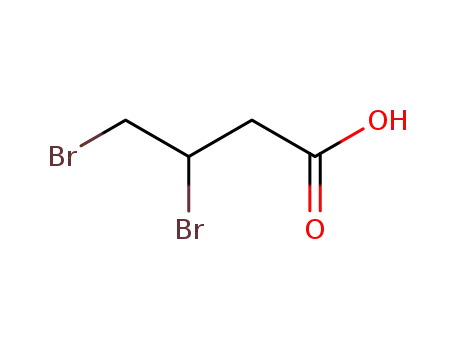
(±)-3,4-dibromobutanoic acid
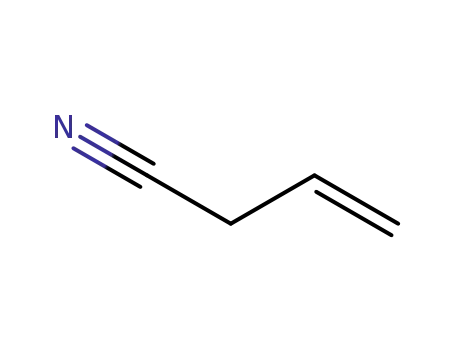
but-3-enenitrile
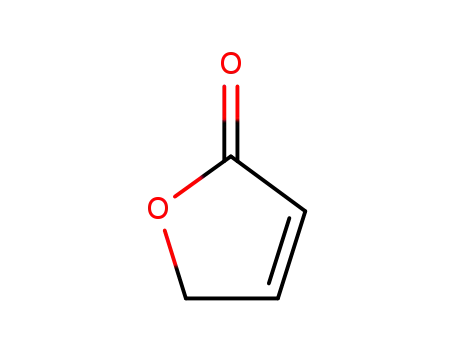
2-buten-4-olide
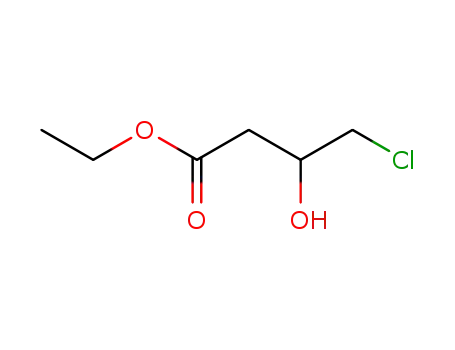
ethyl 4-chloro-3-hydroxybutanoate

3,4-dihydroxybutyric acid
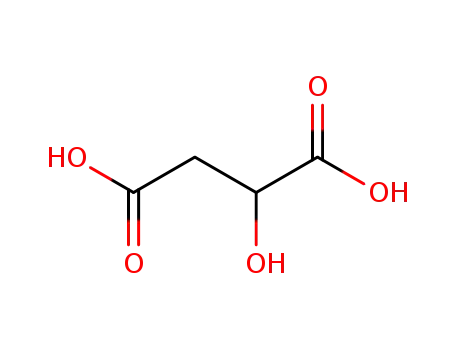
malic acid
CAS:119356-77-3
CAS:868844-74-0
CAS:307297-39-8
CAS:129954-34-3